Introduction
The aorta is directly connected to the heart. Diseases of the aorta include aortic dilatation, aortic aneurysm, and aortic dissection, and several complications may arise after corrective surgery to treat these conditions [
1,
2]. Paraplegia is a notable complication of aortic surgery that warrants careful evaluation due to its potential to diminish a patient’s quality of life and independence in daily activities. The reported prevalence of this complication varies in the literature, with rates ranging from 0.3% to 38% [
1,
3]. Spinal cord ischemia has been suggested as the most common cause of lower-limb weakness after aortic surgery. During aortic surgery, the proximal descending aorta is clamped to correct pathologies in the thoracic aorta, disrupting blood flow to the distal part of the aorta. If the clamping time is prolonged, ischemia or infarction of the distal portion may occur. This area includes several radicular arteries, most notably the artery of Adamkiewicz, that supply oxygen to the thoracic spinal cord. Consequently, prolonged clamping can result in paraplegia due to ischemic injury of the thoracic spinal cord [
3,
4].
However, paraplegia following aortic surgery may not always be attributable to ischemic spinal cord injury [
4,
5]. In the present case, features of peripheral nerve injury were observed, including lumbosacral plexopathy and femoral neuropathy. Ischemic lumbosacral plexopathy with paraplegia can occur after surgery of the distal abdominal aorta or iliac artery [
4,
6]. However, no case reports have been published on lumbosacral plexopathy following proximal aortic surgery. Therefore, this case report is intended to discuss the occurrence of bilateral ischemic lumbosacral plexopathy after ascending aorta and total arch replacement, which is classified as proximal aortic surgery.
Case Report
A 76-year-old woman presented with dyspnea on exertion that had begun 2 years prior. Echocardiography performed in February 2019 revealed severe aortic regurgitation and ascending aortic dilatation. On February 14, 2020, due to progressive symptoms and aggravated aortic regurgitation, the patient underwent aortic valve replacement with ascending aorta and total arch replacement. The following day, venoarterial extracorporeal membrane oxygenation (VA-ECMO) was administered to the right thigh due to cardiac tamponade. The patient subsequently underwent two hematoma removal procedures to address bleeding at the cardiac surgery site, after which she was admitted to the intensive care unit. On February 22, which was postoperative day (POD) 8, the patient regained consciousness after sedatives were tapered. However, she was unable to move her bilateral lower extremities. On the same day, ischemic colitis was confirmed via sigmoidoscopy. A physical examination revealed that the patient’s consciousness and orientation were intact; however, the muscle strength of her bilateral lower extremities was assessed as grade 0, and sensation was impaired below both thighs. The patient’s deep tendon reflexes were reduced, but no abnormalities were observed in the upper extremities, and no pathological reflexes were induced.
VA-ECMO was removed on POD 17. Subsequent electroencephalography (EEG) and brain computed tomography (CT) scans performed on POD 19 revealed no abnormalities. Once the patient’s condition stabilized, she was transferred from the intensive care unit to the general ward on POD 51. Two days later, on POD 53, thoracic diffusion-weighted magnetic resonance imaging (MRI) indicated no signal changes suggestive of ischemic damage to the spinal cord. In a nerve conduction study (NCS) performed on POD 54, no response was observed in either the motor (femoral, peroneal, and tibial) or the sensory (lateral femoral cutaneous, sural, saphenous, and superficial peroneal) nerves in the bilateral lower limbs (
Table 1).
Therefore, due to the potential for bilateral lumbosacral plexopathy, conservative treatment, and rehabilitation were initiated. This included passive range-of-motion exercises, standing training via a tilting table, ergometer use, and gait training with a lifting device. To investigate the persistent paraplegia, electromyography (EMG) was performed on POD 73. However, this evaluation was limited by poor patient cooperation. Based on the study, all muscles examined (including the vastus medialis, iliopsoas, and right tibialis anterior muscles) displayed diffuse abnormal spontaneous activities (specifically, positive sharp waves and fibrillation potentials), and no motor unit action potentials were observed (
Tables 2,
3). On POD 197, T2-weighted lumbosacral plexus MRI revealed diffuse increased signal intensity in the right lumbar third, fourth, and fifth nerve roots, as well as both lumbosacral plexuses. This suggested diffuse swelling of the nerve. No abnormal findings were observed in the spinal cord at the lumbosacral level (
Fig. 1). At discharge, no improvement in muscle strength was noted for any muscle group. The patient continued her treatment at a hospital in her hometown.
Discussion
The lumbosacral plexus is composed of the lumbar plexus, which includes the ventral branches of the L1-L4 nerve roots, and the sacral plexus, which includes the ventral branches of the L4 and L5 and the S1-S3 nerve roots [
7]. The lumbar plexus gives rise to nerves such as the femoral, obturator, and lateral femoral cutaneous nerves, which are responsible for movement and sensation in the upper parts of the lower limbs. The sacral plexus primarily consists of the sciatic nerve, which branches into the peroneal and tibial nerves, responsible for movement and sensation in the distal lower limbs. Lumbosacral plexopathy can result from both structural and non-structural lesions in this area. Structural issues include hematoma, aneurysm, and trauma, while non-structural issues encompass ischemic injury, surgery-related stretch injury, inflammation, and radiation injury [
7]. In most cases, plexopathy presents unilaterally, with bilateral involvement primarily reported following radiation therapy [
8]. Ischemic lumbosacral plexopathy is not widely recognized due to its low incidence rate, but bilateral symptoms have been reported in some postoperative cases, including bilateral aortic iliac artery occlusion and descending aortic dissection [
3,
9,
10].
Aortic surgery causes substantial hemodynamic fluctuations and can impact the human body in numerous ways. A previous study suggested that the risk of postoperative complications increases when the aortic cross-clamping (ACC) time exceeds 150 minutes [
11]. Therefore, when evaluating paraplegia after surgery, it is crucial to conduct a thorough evaluation of the corticospinal tract structures originating from the primary motor cortex [
9]. The cerebral cortex, spinal cord, lumbosacral nerve root, lumbosacral plexus, and other peripheral nerves should also be evaluated [
10]. While no systematic reviews are available on complications after aortic surgery, several studies have reported incidence rates including stroke in 1.2% to 17% of patients, spinal cord injuries in 0.25% to 11.1% of cases, lumbosacral plexopathy in 0% to 0.3% of cases, and peripheral nerve injuries in 3.4% of cases [
2,
4-
6,
12-
14]. As demonstrated in the present case, brain CT and EEG can be utilized to detect cerebral abnormalities, and MRI scans of multiple sites can be performed to confirm ischemic spinal cord injuries commonly seen in aortic surgery. Additionally, NCS and EMG can be used to assess the condition from the nerve root to the peripheral nerve, helping to identify the location of the lesion.
In this case, abnormal findings were observed on the sensory NCS of both lower limbs. This suggests a potential lesion in the lumbosacral plexus or peripheral nerve, distal to the dorsal root ganglion [
7]. Subsequent EMG revealed abnormalities in all nerves, suggesting lumbosacral plexopathy rather than diffuse mononeuropathies [
7]. Clinically, paraplegia results from extensive ischemic injury that occurs distal to the site of proximal aortic surgery. In the present case, the total ACC time during the operation with cardiopulmonary bypass was 266 minutes. Severe hypotension ensued postoperatively due to cardiac tamponade; this necessitated two additional hematoma removal procedures, and accompanying ischemic colitis was confirmed. MRI revealed a diffuse edematous lesion on the bilateral lumbosacral plexus, which may have been caused by ischemic neuropathy.
This report presents a case of bilateral lumbosacral plexopathy with an intact spinal cord following proximal aortic surgery, which had not been previously documented. This case underscores the need to consider the possibility of lumbosacral plexopathy and ischemic spinal cord injury in patients who experience paraplegia after proximal aortic surgery. Furthermore, given the risk of these complications, it is advisable to employ more active intraoperative neurophysiological monitoring during aortic surgery.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Fig. 1.
Magnetic resonance images of the lumbosacral plexus, obtained on postoperative day 197, from a patient who developed paraplegia after proximal aortic surgery. T2-weighted images reveal (A, B) diffuse swelling of the right L3, L4, and L5 nerve roots and lumbar plexus (thick arrows), as well as (C, D) suspicious increased T2 signal intensity of the left lumbar plexus, indicating bilateral lumbar plexopathy (arrows). (E) No signal changes were observed that would indicate a spinal cord lesion.
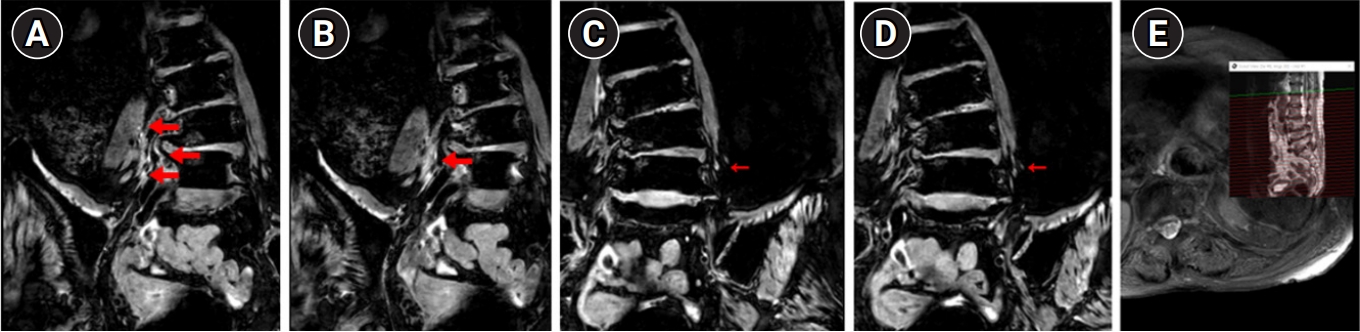
Table 1.
Nerve Conduction Study on Postoperative Day 51 Suggesting Bilateral Lumbosacral Plexopathy
|
Variable |
Stimulation site |
Recording site |
Latency (m/sec) |
Amplitude (µV) |
Velocity (m/sec) |
|
Sensory |
|
|
|
|
|
|
Lt. median |
Wrist |
Third finger |
3.70 |
21.0 |
|
|
Lt. ulnar |
Wrist |
Fifth finger |
2.70 |
17.5 |
|
|
Rt. superficial peroneal |
Lateral leg |
Ankle |
NR |
NR |
|
|
Lt. superficial peroneal |
Lateral leg |
Ankle |
NR |
NR |
|
|
Rt. sural |
Calf |
LM |
NR |
NR |
|
|
Lt. sural |
Calf |
LM |
NR |
NR |
|
|
Rt. saphenous |
Calf |
MM |
NR |
NR |
|
|
Lt. saphenous |
Calf |
MM |
NR |
NR |
|
|
Rt. LFCN |
Inguinal ligament |
Lateral thigh |
NR |
NR |
|
|
Lt. LFCN |
Inguinal ligament |
Lateral thigh |
NR |
NR |
|
|
Motor |
|
|
|
|
|
|
Lt. median |
Wrist |
APB |
4.60*
|
9.6 |
|
|
Elbow |
APB |
8.10 |
8.9 |
54.2 |
|
Lt. ulnar |
Wrist |
ADM |
2.20 |
9.4 |
|
|
Elbow |
ADM |
5.30 |
8.3 |
59.6 |
|
Rt. deep peroneal |
Ankle |
EDB |
NR |
NR |
|
|
Fibular head |
EDB |
NR |
NR |
|
|
Lt. deep peroneal |
Ankle |
EDB |
NR |
NR |
|
|
Fibular head |
EDB |
NR |
NR |
|
|
Rt. femoral |
Inguinal ligament |
VM |
NR |
NR |
|
|
Lt. femoral |
Inguinal ligament |
VM |
NR |
NR |
|
|
Rt. tibial |
Ankle |
AH |
NR |
NR |
|
|
Knee |
AH |
NR |
NR |
|
|
Lt. tibial |
Ankle |
AH |
NR |
NR |
|
|
Knee |
AH |
NR |
NR |
|
Table 2.
Nerve Conduction Study* on Postoperative Day 73 Corresponding to Bilateral Lumbosacral Plexopathy
|
Variable |
Stimulation site |
Recording site |
Latency (m/sec) |
Amplitude (µV) |
Velocity (m/sec) |
|
Sensory |
|
|
|
|
|
|
Rt. superficial peroneal |
Lateral leg |
Ankle |
NR |
NR |
NR |
|
Motor |
|
|
|
|
|
|
Rt. deep peroneal |
Ankle |
EDB |
NR |
NR |
NR |
|
Fibular head |
EDB |
NR |
NR |
NR |
|
Lt. deep peroneal |
Ankle |
EDB |
NR |
NR |
NR |
|
Fibular head |
EDB |
NR |
NR |
NR |
|
Rt. femoral |
Inguinal ligament |
VM |
NR |
NR |
NR |
|
Lt. femoral |
Inguinal ligament |
VM |
NR |
NR |
NR |
Table 3.
Electromyography Study on Postoperative Day 73 Corresponding to Bilateral Lumbosacral Plexopathy
|
Muscle |
Spontaneous |
MUAP |
Recruitment pattern |
Interference pattern |
|
Fib |
PSW |
Amplitude |
Duration |
Phase |
|
Rt. tibialis anterior |
3+ |
3+ |
|
No MUAP |
|
|
|
|
Rt. vastus medialis |
2+ |
2+ |
|
No MUAP |
|
|
|
|
Rt. iliopsoas |
2+ |
2+ |
|
No MUAP |
|
|
|
|
Lt. vastus medialis |
1+ |
1+ |
|
No MUAP |
|
|
|
|
Lt. iliopsoas |
1+ |
1+ |
|
No MUAP |
|
|
|
|
Rt. first dorsal interossei |
None |
None |
NL |
NL |
NL |
NL |
NL |
|
Rt. deltoid |
None |
None |
NL |
NL |
NL |
NL |
NL |
REFERENCES
1. Crawford ES, Crawford JL, Safi HJ, Coselli JS, Hess KR, Brooks B, et al: Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg 1986;3:389-404.


2. Melissano G, Tshomba Y, Bertoglio L, Rinaldi E, Chiesa R: Analysis of stroke after TEVAR involving the aortic arch. Eur J Vasc Endovasc Surg 2012;43:269-275.


3. Shenaq SA, Svensson LG: Paraplegia following aortic surgery. J Cardiothorac Vasc Anesth 1993;7:81-94.


4. Gloviczki P, Cross SA, Stanson AW, Carmichael SW, Bower TC, Pairolero PC, et al: Ischemic injury to the spinal cord or lumbosacral plexus after aorto-iliac reconstruction. Am J Surg 1991;162:131-136.


5. Boontje AH, Haaxma R: Femoral neuropathy as a complication of aortic surgery. J Cardiovasc Surg (Torino) 1987;28:286-289.

7. Preston DC, Shapiro BE: Lumbosacral plexopathy. In: Preston DC, Shapiro BE, editors. Electromyography and neuromuscular disorders: clinical-electrophysiologic-ultrasound correlations. 4th ed. Philadelphia: Elsevier; 2020, pp622-640.
9. Álvarez M, Lucente G, Martínez L, Almendrote M, Ramos A, Broto J, et al: Paraplegia following type B acute aortic dissection can spare the spinal cord. Ann Vasc Surg 2021;70:569.

10. Kim H, Kang SH, Kim DK, Seo KM, Kim TJ, Hong J: Bilateral ischemic lumbosacral plexopathy from chronic aortoiliac occlusion presenting with progressive paraplegia. J Vasc Surg 2014;59:241-243.


12. Conrad MF, Ye JY, Chung TK, Davison JK, Cambria RP: Spinal cord complications after thoracic aortic surgery: long-term survival and functional status varies with deficit severity. J Vasc Surg 2008;48:47-53.


13. Szilagyi DE, Hageman JH, Smith RF, Elliott JP: Spinal cord damage in surgery of the abdominal aorta. Surgery 1978;83:38-56.











