Electrodiagnosis of Multiple Cranial Neuropathies and Lumbosacral Polyradiculopathy in Tuberculous Meningitis: A Case Report
Article information
Abstract
Cranial neuropathy and radiculopathy are common complications of tuberculous meningitis. However, the co-occurrence of these complications and the corresponding electrodiagnostic findings have rarely been reported. We report a patient diagnosed with tuberculous meningitis who presented with difficulty in smelling, facial paralysis, hearing loss, and severe weakness in the bilateral lower extremities. A neurological examination confirmed impaired function of the olfactory nerves. Electrodiagnostic studies, including a nerve conduction study, electromyography, and brainstem auditory evoked potential, revealed multiple cranial neuropathies involving the left facial nerve and bilateral vestibulocochlear nerves, as well as bilateral lumbosacral polyradiculopathies. In patients with tuberculous meningitis, multiple cranial neuropathies and polyradiculopathies can occur simultaneously as complications, and electrodiagnostic studies can enable an accurate diagnosis of these complications and an assessment of their severity.
Introduction
Cranial neuropathy and radiculopathy can occur as complications of tuberculous meningitis [1-3]. However, the co-occurrence of multiple cranial neuropathies and polyradiculopathies has rarely been reported. Furthermore, to our knowledge, studies reporting the electrodiagnostic findings of these complications are extremely rare [4]. Herein, we report a case of a patient diagnosed with tuberculous meningitis with patchy involvement of multiple cranial nerves and lumbosacral polyradiculopathy.
Case Report
A 61-year-old woman visited a university hospital 4 hours after the onset of fever. Five months prior, she had been hospitalized for a fever that lasted 6 months. Her only underlying disease was dyslipidemia. On admission, arthralgia was observed in the bilateral shoulder, wrist, metacarpophalangeal, proximal interphalangeal, knee, and ankle joints, without skin lesions. She underwent abdomen-pelvis and chest computed tomography which showed no infectious fever focus. A bone scan revealed arthritis in multiple joints, including the bilateral shoulder, elbow, wrist, and knee joints (Fig. 1), and whole-body positron emission tomography suggested reactive hypersplenism and inflammatory reactive lymph nodes. Serum laboratory testing showed leukocytosis (16,740/mm3) with neutrophil dominance (94.7%), a high C-reactive protein (CRP) level (18.77 mg/dL), a high erythrocyte sedimentation rate (ESR, 102 mm/h) and positive rheumatoid factor. Anti-cyclic citrullinated peptide and anti-nuclear antibody were negative, and creatine kinase (108 IU/L) was not elevated. Based on these results, the patient was diagnosed with seropositive rheumatoid arthritis or adult-onset Still’s disease. She was prescribed methotrexate, hydroxychloroquine, cyclosporine, prednisolone, and cyclophosphamide based on these diagnostic possibilities.
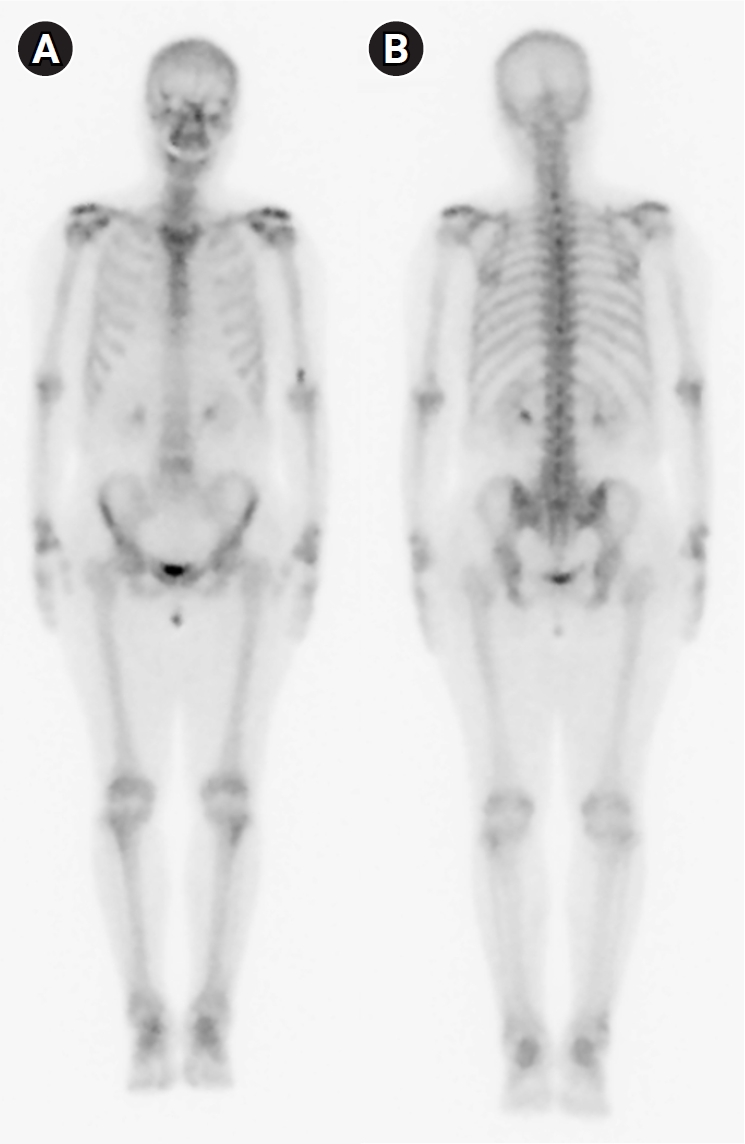
Bone scan (A: anterior view; B: posterior view) performed during the previous hospitalization, showing mildly increased uptake in the bilateral shoulder, elbow, wrist, and knee joints.
Afterward, she had a mild fever; however, 5 months later, a more severe fever of > 38°C occurred; this was accompanied by a confused mental state, vomiting, and general weakness. On a neurological examination, she was confused and her orientation to time and place was impaired. She also showed bilateral hearing impairment and left facial palsy of the peripheral type with House-Brackmann grade 2. Appropriate oral feeding was impossible because of her confused state; therefore, a nasogastric tube was inserted. She had bilateral flaccid weakness of the lower extremities (grade 1 of 5 on the Medical Research Council scale). In addition, severe neuropathic pain was present in her bilateral lower extremities.
Brain magnetic resonance imaging (MRI) revealed diffuse sulcal enhancement with small nodular enhancing foci in a gadolinium-enhanced T1 sequence, suggesting a diagnosis of meningoencephalitis associated with tuberculosis, neurosyphilis, or granulomatous inflammation. Whole-spine MRI showed extensive irregular leptomeningeal thickening and enhancement along the spinal cord, as well as cauda equina (Fig. 2A-D).
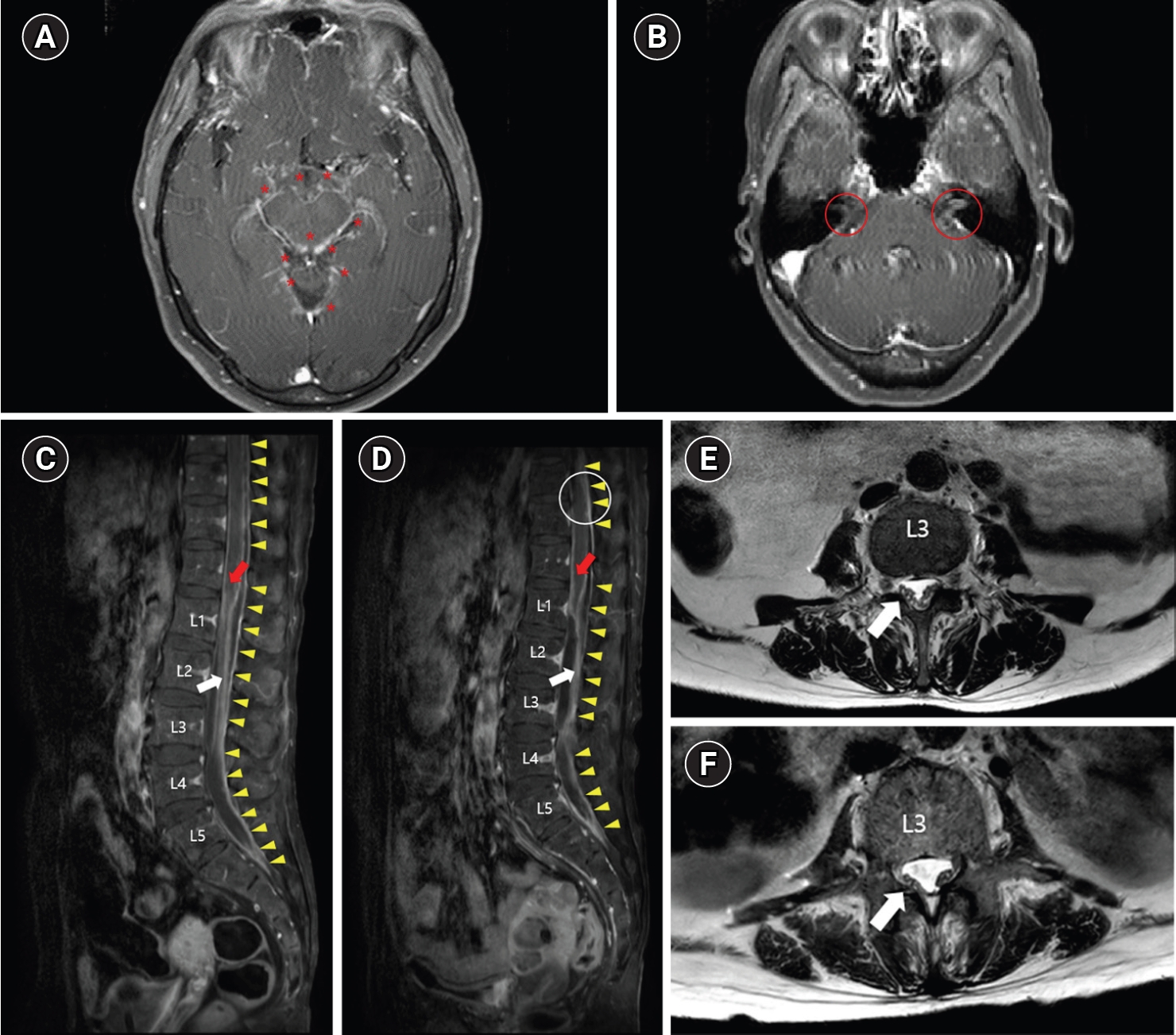
Brain (A, B) and spine (C, D, E, F) magnetic resonance imaging (MRI) of the patient. (A) Gadolinium-enhanced T1 axial view at the midbrain level. Asterisks (*) indicate small nodular enhancing foci of the diffusely enhanced meninx. (B) Gadolinium-enhanced T1 axial view at the pons level. Red circles indicate the right and left facial-vestibulocochlear nerve complexes with perineural enhancement. (C) Gadolinium-enhanced T1 sagittal view of the first spine MRI. The red arrow indicates conus medullaris with surface enhancement, and the white arrow indicates cauda equina with irregular leptomeningeal thickening and enhancement. Yellow arrowheads indicate dural enhancement along the spinal canal. (D) T2 axial view at the L3 level of the first spine MRI. The white arrow indicates the cauda equina with irregular leptomeningeal thickening and enhancement at the L3 level. (E) Gadolinium-enhanced T1 sagittal view of the follow-up spine MRI. Compared to the first spine MRI (C), the cauda equina (white arrow) is clumped and adherent to the dural sac, and obliteration of the subarachnoid space (white circle) was observed. (F) T2 axial view at the L3 level of the follow-up spine MRI. The white arrow indicates the cauda equina with more adhesion to the dural sac than the first spine MRI (D).
Serum complete blood counts showed a normal white blood cell count (9,410/mm3) with a high neutrophil proportion (96.6%). Elevation of the CRP level (2.85 mg/L) and ESR (49 mm/h) was observed, but the results were lower than those observed in the previous hospitalization. A cerebrospinal fluid (CSF) study revealed a high adenosine deaminase level (27 IU/L) with pleocytosis (456 white blood cells per mm3) of mononuclear cell dominance (87.1%) and a high protein level (3,848 mg/dL), suggestive of tuberculous infection despite negative culture results for Mycobacterium tuberculosis. An absence of arthralgia aggravation and salmon-colored rash, combined with a normal liver function test, suggested that a flare-up of adult-onset Still’s disease was unlikely. On the day the patient was transferred from the emergency room to the Department of Neurology, which was 2 days after the onset, she commenced taking anti-tubercular medications (rifampin, isoniazid, pyrazinamide, and ethambutol) for tuberculous meningoencephalitis. Follow-up spine MRI performed 26 days after the first spine MRI revealed clumped and adherent cauda equina to the dural sac and multifocal obliteration of the subarachnoid space, suggestive of adhesive arachnoiditis (Fig. 2E, F). A videofluoroscopic swallowing study performed 1 month after the onset showed no definite penetration or aspiration for all tested foods; thus, the nasogastric tube was removed. After the removal of the nasogastric tube, she had no difficulty eating a regular diet.
After acute management in the Department of Neurology, the patient was transferred to the Department of Rehabilitation Medicine, 35 days after the onset. A follow-up neurologic examination revealed impairment of olfactory function, left peripheral type facial palsy, bilateral hearing impairment, and severe bilateral lower-extremity weakness. The muscle strength of the right and left lower extremities was 0/0 for hip flexors, 0/1 for knee extensors and knee flexors, and 1/1 for ankle dorsiflexors, big toe extensors, and ankle plantar flexors. Knee jerks were decreased bilaterally. In addition, voluntary anal contraction was absent, and the sensory function of the S4–5 dermatome was impaired. A Foley catheter that had been inserted in the emergency room was removed, but self-urination was not possible and clean intermittent catheterization was performed.
One day after transfer, comprehensive electrodiagnostic studies were performed to evaluate the involvement of the cranial nerves and peripheral nervous system.
In a motor nerve conduction study (NCS), the amplitudes of bilateral peroneal, right tibial, and left facial compound muscle action potentials were diminished (Table 1). Sensory NCS showed no abnormalities, and F-waves of the bilateral peroneal nerves and H-reflex tests for bilateral tibial nerves showed no response. The blink reflex test showed no response on the left side.
Electromyography showed denervation potentials in several facial and lower-extremity muscles (Table 2). Motor unit action potential (MUAP) analyses showed no MUAP activity or discrete interference pattern in the lower-extremity muscles and a discrete to reduced pattern in the left facial muscles. Brainstem auditory evoked potential testing, which was performed to evaluate auditory function, elicited no response bilaterally.
Overall, the findings of the electrodiagnostic study indicated cranial neuropathies involving the left facial nerve, bilateral vestibulocochlear nerves, and bilateral lumbosacral polyradiculopathies involving the nerve roots at and below the L2 level; this was in addition to the impairment of olfactory nerves found in the neurologic examination.
During the patient’s 3-week stay in the Department of Rehabilitation Medicine, rehabilitation therapies, including range-of-motion exercises, strengthening exercises, and functional electrical stimulation, were provided; however, lower-extremity motor power and gait function did not show significant improvements. Two months after discharge from the hospital, lower-extremity weakness, gait ability, and urinary function were unimproved, while left facial palsy and hearing impairment of both ears showed partial improvement.
The study was approved by the institute review board of Seoul National University Bundang Hospital (IRB no: B-2208-773-703) and written informed consent was obtained from the patient.
Discussion
This article reports a patient diagnosed with tuberculous meningoencephalitis presenting with difficulty in smelling, facial palsy, hearing loss, and severe lower-extremity weakness. An electrodiagnostic examination demonstrated patchy involvement of multiple cranial nerves and lumbosacral polyradiculopathies.
In tuberculous meningitis, cranial neuropathy and radiculopathy are common complications; however, few studies have reported cases of the simultaneous occurrence of multiple cranial neuropathies and polyradiculopathies [4].
The prevalence of cranial nerve involvement in tuberculous meningitis ranges from 14.8% to 38% [1,2]. The most frequently involved cranial nerves are the oculomotor, abducens, optic, facial, and vestibulocochlear nerves [1,2]. In tuberculous meningitis, the thick gelatinous exudates, which are especially copious in the brain’s basilar regions, can entrap the cranial nerve trunks, or increased intracranial pressure can damage them [1]. In our patient, perineural enhancement of the facial-vestibulocochlear nerve complex was observed on brain MRI (Fig. 2B). Together with the electrodiagnostic study results, this suggested injury of these cranial nerves. Cranial nerve involvement has been reported to be associated with the age of onset (< 25 years), hydrocephalus, disability at presentation (modified Rankin Scale [mRS] > 2), altered sensorium, and CSF profile (protein > 250 mg/dL, cell count > 100) [1]. Our patient presented severe disability (mRS 5), altered sensorium (decreased sensation in the S4–5 dermatome), and CSF abnormalities with high cell counts and protein levels. Cranial nerve involvement in tuberculous meningitis is associated with poor outcomes, such as mRS > 2, presence of neurologic sequelae, or decreased consciousness [1,5]; therefore, a precise evaluation and diagnosis of cranial neuropathy are important.
Few studies have reported electrodiagnostic tests performed to diagnose radiculopathy and evaluate disease severity [3,6]. Tuberculous arachnoiditis is generally the cause of radiculomyelopathy associated with tuberculous meningitis. This most characteristic spinal complication is produced by the thick inflammatory exudates surrounding the spinal cord, as in cranial nerve involvement. The thick gelatinous exudates fill the subarachnoid space, encasing the spinal cord and emerging nerve roots, and making them inflamed. The exudates, in late stages, may eventually lead to adhesion and atrophy of nerve roots [3]. The initial spine MRI of our patient showed extensive leptomeningeal enhancement reflective of subarachnoid exudate affecting the spinal cord and cauda equina, and the follow-up MRI demonstrated the development of adhesive arachnoiditis (Fig. 2C-F). A high CSF protein level (> 250 mg/dL) and a low modified Barthel index (< 12) have been reported to be associated with spinal cord and nerve root involvement [6]. The prognosis of tuberculous meningitis with spinal cord or nerve root involvement is not predictable; however, it has been reported that sequelae developed in 14.2% of patients, and poor outcomes are associated with spinal cord atrophy, syringomyelia, and a multiplicity of complications, such as radiculomyelitis, spinal tuberculoma, or spinal abscess [3].
This case report describes the electrodiagnostic findings of polyradiculopathy and multiple cranial neuropathies caused by tuberculous meningitis, along with the imaging and laboratory findings and the patient’s clinical outcomes. Further publications presenting electrodiagnostic findings in tuberculous meningitis may confirm the prognostic value of electrodiagnostic studies in patients with tuberculous meningitis.
In conclusion, multiple cranial neuropathies and polyradiculopathies can occur simultaneously in patients with tuberculous meningitis, and comprehensive electrodiagnostic studies should be considered to evaluate these complications.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.


