Acute Transverse Myelitis after Varicella-Zoster Virus Infection in an Immunocompetent Patient: A Case Report
Article information
Abstract
Herpes zoster is caused by reactivated varicella-zoster virus (VZV) and characterized by a painful skin rash with vesicles that affects the adjacent dermatomes. Transverse myelitis is a rare complication of VZV infection that may even occur in immunocompetent patients. Here, we report a 60-year-old male patient admitted with right lower-extremity weakness and hypesthesia. Spine magnetic resonance imaging (MRI) revealed transverse myelitis at the C3–C4 and T6 levels. A month prior to admission, the patient was diagnosed with herpes zoster involving the right T2–T4 dermatome. Considering his history of VZV infection, he was treated with intravenous steroids and acyclovir. Eleven days after hospitalization, paraplegia developed to Medical Research Council grade 0/5 despite performing plasmapheresis. MRI confirmed the aggravation of the cord lesion between T4 and T7. Acute transverse myelitis after VZV infection is a rare disease that can cause serious sequelae in immunocompetent patients. Therefore, clinicians should be cautious of this situation.
Introduction
Varicella-zoster virus (VZV) infection is clinically common in otherwise healthy individuals and is characterized by skin rashes and neuropathic pain along the dermatome. After the first infection, the virus becomes latent in the ganglia and reactivates in an immunosuppressed state, causing neurological complications such as postherpetic neuralgia [1,2]. Complications involving the central nervous system such as encephalitis and myelitis, are relatively rare, especially in immunocompetent patients [3]. Here, we report a case of transverse myelitis after VZV infection in an immunocompetent patient.
Case Report
This study was conducted with informed consents of the patient.
A 60-year-old male patient was admitted to our hospital with motor weakness and sensory dysfunction of the right lower extremity. On admission, his vital signs were stable, and he showed normal cranial nerve and cerebellar function. The muscle strength of the upper and left lower extremities was normal, but that of the right lower limb decreased to Medical Research Council (MRC) grade 4/5. Sensation in his right lower extremity also decreased. Despite the motor weakness and sensory dysfunction, he was able to walk independently. Deep tendon reflexes (DTRs) were normal in both upper extremities, but were increased in both the knees and ankles. The Babinski sign was observed on both sides, and the Hoffman sign was observed on the right upper limb.
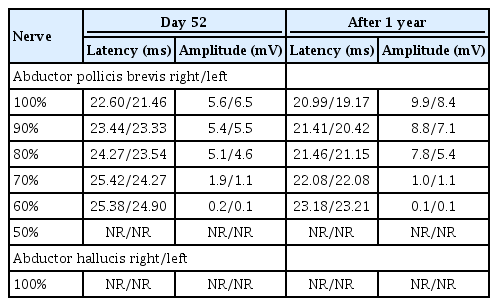
Magnetic resonance imaging (MRI) indicated nodular enhancement of the spinal cord at the right C3–C4 level (Fig. 1) and hyperintensity of the spinal cord at T6 on T2-weighted imaging (Fig. 2). No other specific brain lesion was observed on brain MRI.
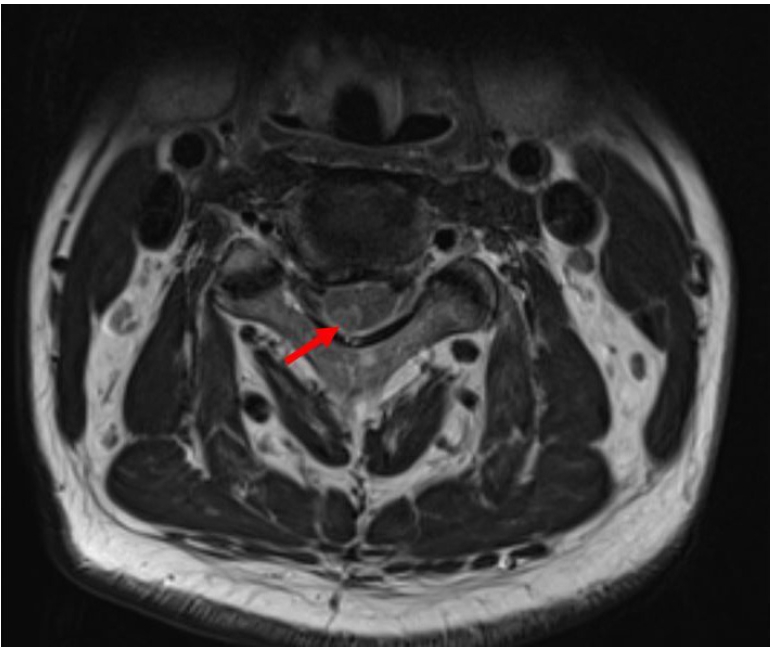
Image showing the nodular enhancement of right C3-C4 level (arrow) on axial T2 weighted magnetic resonance imaging.
(A) Image showing high signal intensity between C3 and C4 level (arrows) at day 2 on T2 weight magnetic resonance imaging (MRI). (B) Image showing high signal intensity on T6 level at day 2 on T2 weight MRI.
The laboratory findings for complete blood cell count, liver function, kidney function, infection markers, and urine analysis were normal. A cerebrospinal fluid (CSF) study showed an increase in protein (36 mg/dL) and myelin basic protein (4.8 μg/L) levels with an opening pressure of 18 cmH2O. All bacterial antigens, polymerase chain reaction (PCR) tests, culture studies, and antigen tests, including VZV, were negative (Table 1). All autoimmune antibody tests performed to exclude autoimmune myelitis were also negative (Table 2).

Culture Study, Bacterial and Viral Polymerase Chain Reaction Study and Antibody Test for Cerebrospinal Fluid
A month prior to admission, the patient had a painful erythematous vesicle on the right T2–T4 dermatome. He was diagnosed with VZV infection of the right T2–T4 dermatome based on clinical symptoms and treated with oral valacyclovir (1,000 mg) for 7 days. After 5 days of valacyclovir administration, the vesicles and pain improved. However, erythematous skin lesions persisted for more than 1 month until his hospitalization for motor weakness and sensory dysfunction. Upon admission, an erythematous skin lesion measuring 4 to 5 cm was observed on the right T2–T4 dermatome.
Considering the history of VZV infection, we diagnosed the patient with acute transverse myelitis (ATM) caused by VZV and started acyclovir (2,100 mg/day) for 14 days, along with steroid pulse therapy (intravenous methylprednisolone [1,000 mg] for 5 days and oral prednisolone 300 mg for 5 days, tapered for 4 days).
On the second day of hospitalization, a somatosensory potential (SEP) test was performed. The SEPs of the left median nerve and bilateral tibial nerves were normal. However, the SEP of the right median nerve had decreased by more than half compared with that on the left side.
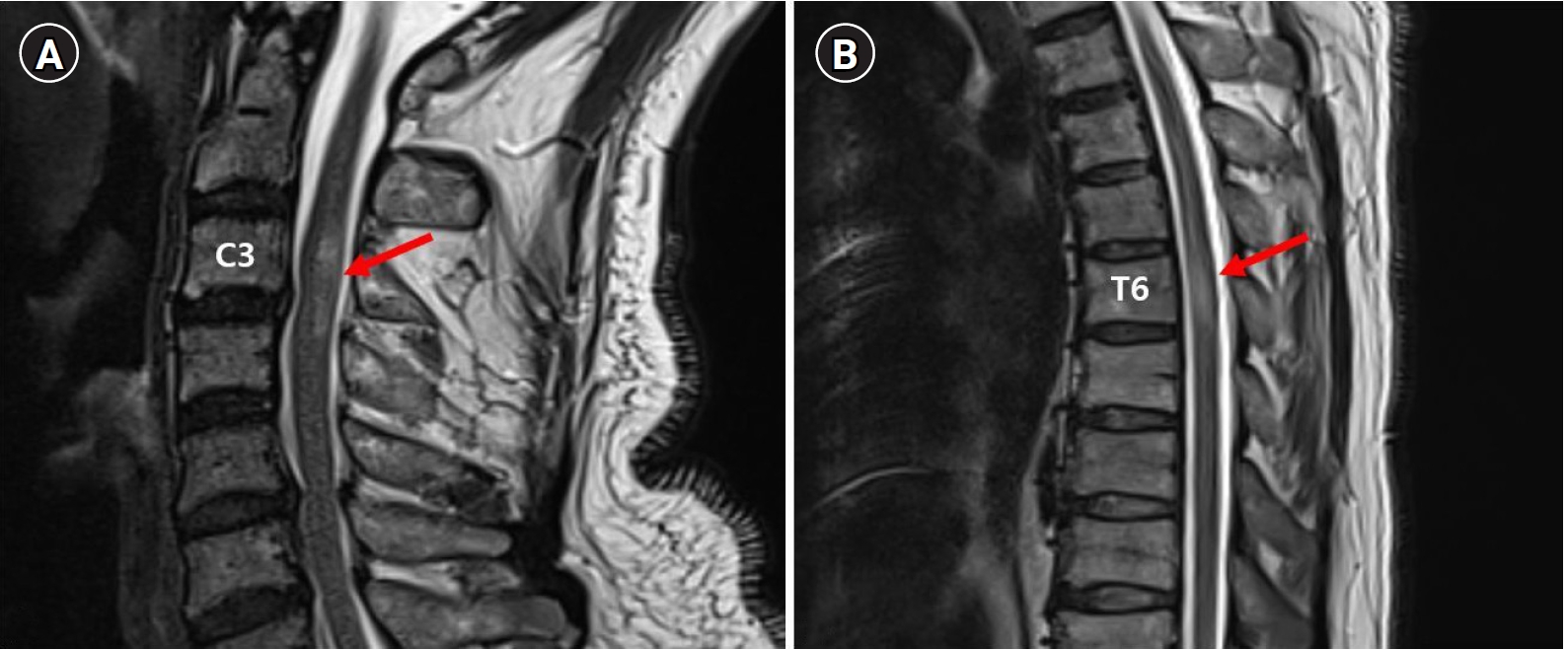
On the third day of hospitalization, the patient complained of voiding difficulty. A Foley catheter was inserted, and 950 mL of urine was drained. His lower extremity muscle strength started to deteriorate gradually. On the seventh day of hospitalization, the muscle strength of the lower extremities decreased to MRC grade 2/5. The patient complained of decreased sensation of the C3–C4 dermatomes of the right upper extremity, and no sensation was felt at the dermatome lower than the T6 level. Cervical and thoracic spine MRI, which was performed on the 10th day of hospitalization, confirmed the aggravation of the cord lesion between T4 and T7 (Fig. 3). Nevertheless, the muscle weakness of the lower extremities worsened to MRC grade 0/5, although plasmapheresis was performed. The patient lost perianal sensation and voluntary contraction of the anal sphincter, and the DTRs of the lower extremities disappeared on the 11th day of hospitalization. Upper and lower extremity nerve conduction studies performed on the 13th day of hospitalization were normal. However, needle electromyography (EMG) showed increased insertional activity and positive sharp waves on both sides of the gastrocnemius, tibialis anterior, peroneus longus, vastus medialis, tensor fasciae latae, and thoracic paraspinal muscles, which were compatible with complete thoracic cord lesions [4]. There were no abnormal findings in the muscles of the right upper extremity, such as the biceps, abductor pollicis brevis, and first dorsal interosseous muscle.
(A) Image showing high signal intensity between C3 and C4 level (arrow) at day 10 on T2 weight magnetic resonance imaging (MRI). (B) Image showing aggravation of lesion between T4 and T7 (arrow) at day 10 on T2 weighted MRI.
After progressing to complete paraplegia, additional plasmapheresis was performed seven times with steroid therapy. However, there was no recovery of motor or sensory function. Motor evoked potential (MEP) and SEP tests were performed to predict the prognosis. The MEP and SEP tests of the lower extremities performed on the 52nd day of hospitalization showed no response (Tables 3, 4), although those of the upper extremities were normal. At that time, the DTRs of both lower extremities showed hyperreflexia, whereas the upper extremity showed normoreflexia.
After 1 year, the patient revisited the outpatient clinic for an evaluation of his disability grade. The muscle strength of the lower extremities remained at MRC grade 0/5, and follow-up MEP and SEP tests of the lower extremities still showed no response (Tables 3, 4).
Discussion
Although VZV infection is common, transverse myelitis caused by VZV is rare, particularly in immunocompetent patients. The pathophysiology of transverse myelitis due to viral infection is known to involve systemic reactions post-infection and the direct invasion of the virus [5,6]. However, the VZV PCR test of CSF is negative in most cases, and the diagnosis is made according to history of viral infection and clinical features [2]. Likewise, in our case, all viral PCR, culture, and antigen tests were negative in the CSF study, and the diagnosis was made considering the patient's history of VZV infection and clinical presentation.
Steroid pulse therapy is the primary treatment option for ATM. In addition, antiviral agents may improve the prognosis of transverse myelitis caused by viral infection. If severe motor weakness is present, plasmapheresis can be performed, along with steroid pulse therapy [7]. Cyclophosphamide is also an option for patients with acute exacerbations [8]. In this case, steroid pulse therapy was administered along with an antiviral agent, but the patient showed no symptom improvement. Cyclophosphamide was not administered in our case, but it could be considered as a treatment option in the future if a patient shows deterioration during conventional steroid pulse and antiviral treatments. Aggravation of the lesion was confirmed on MRI, and dysuria and lower extremity muscle weakness continued to worsen despite steroid and antiviral treatments. We confirmed complete thoracic cord lesions by a nerve conduction study, EMG, and motor- and sensory-evoked potential tests.
In a previous case report, myelitis caused by viral infections in immunocompetent patients showed a good response to steroid treatment and a good prognosis [2,9]. However, in our case, the patient had a poor prognosis, with complete paraplegia. Definitive prognostic factors for ATM remain unclear, but a poor prognosis is associated with complete paraplegia, rapid disease progression, and abnormal SEP findings [10,11]. Necrosis of the motor tract and sensory tract of spinal cord also has a poor prognosis. In contrast, edema and demyelination of the spinal cord tend to resolve quickly and show a better prognosis than necrosis. If more than 6 months have elapsed since onset, EMG can be used to distinguish between necrosis and edema or demyelination. Therefore, the severity of weakness and abnormal spontaneous activity on EMG is the most specific prognostic factor if more than 6 months have elapsed since onset. However, in the early stage, where denervation has not progressed, it is difficult to differentiate between necrosis, edema, and demyelination using EMG. In this situation, MEP and SEP tests could be helpful [12].
In our case, we performed MEP and SEP tests to predict the prognosis on the 52nd day of hospitalization. No response was observed in the MEP and SEP tests performed on the lower extremities. There was also no recovery of motor or sensory function after 1 year. Although the MEP and SEP tests performed on the upper extremity were normal on the 52nd day of hospitalization, the patient complained of sensory dysfunction in the right upper extremity. After 1 year, the function of the upper extremity had fully recovered, possibly in response to the treatment of transverse myelitis with steroids and antiviral agents. However, thoracic lesions were present.
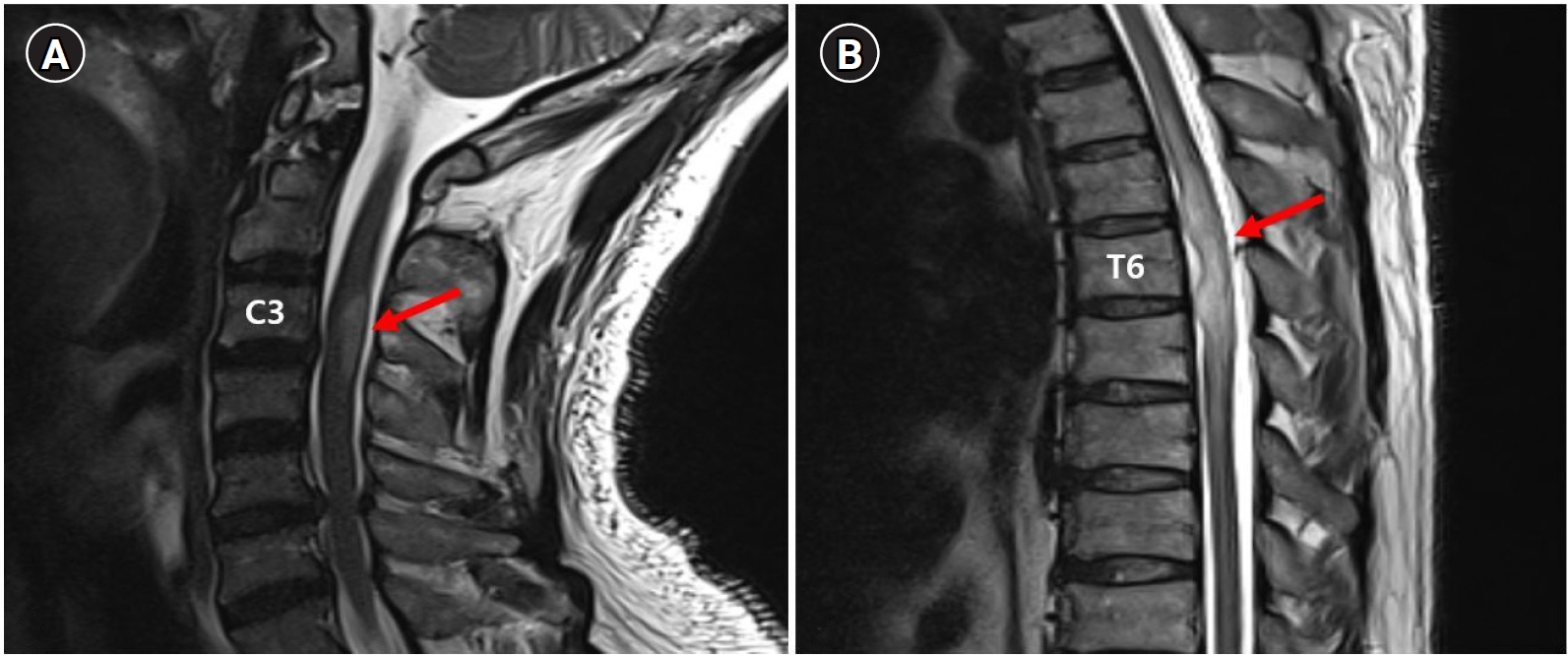
Furthermore, the symptoms worsened rapidly even with steroids and antiviral drugs. This could have been the natural course of the disease, but it is also possible that the lesions were exacerbated by the steroid treatment. Spinal dural arteriovenous fistula (SDAVF) is a rare cause of transverse myelitis. In cases of SDAVF-induced transverse myelitis, symptoms may be exacerbated by steroid treatment and lumbar puncture [13,14]. We diagnosed VZV-induced ATM with a history of VZV infection and myelopathy at a level similar to that of the dermatome, where the zoster was present. However, it was not possible to confirm the diagnosis because the VZV PCR test and CSF culture were negative. The possibility of SDAVF cannot be completely disregarded because thoracic MRI showed the enlargement of spinal blood vessels at the T4–T7 level (Fig. 4) and the exacerbation of lesions after steroid pulse therapy. However, we could not identify specific findings of SDAVF on MRI, such as prominent serpiginous intradural extramedullary flow voids. Magnetic resonance angiography and spinal angiography could have been helpful for the diagnosis, but they were not performed.
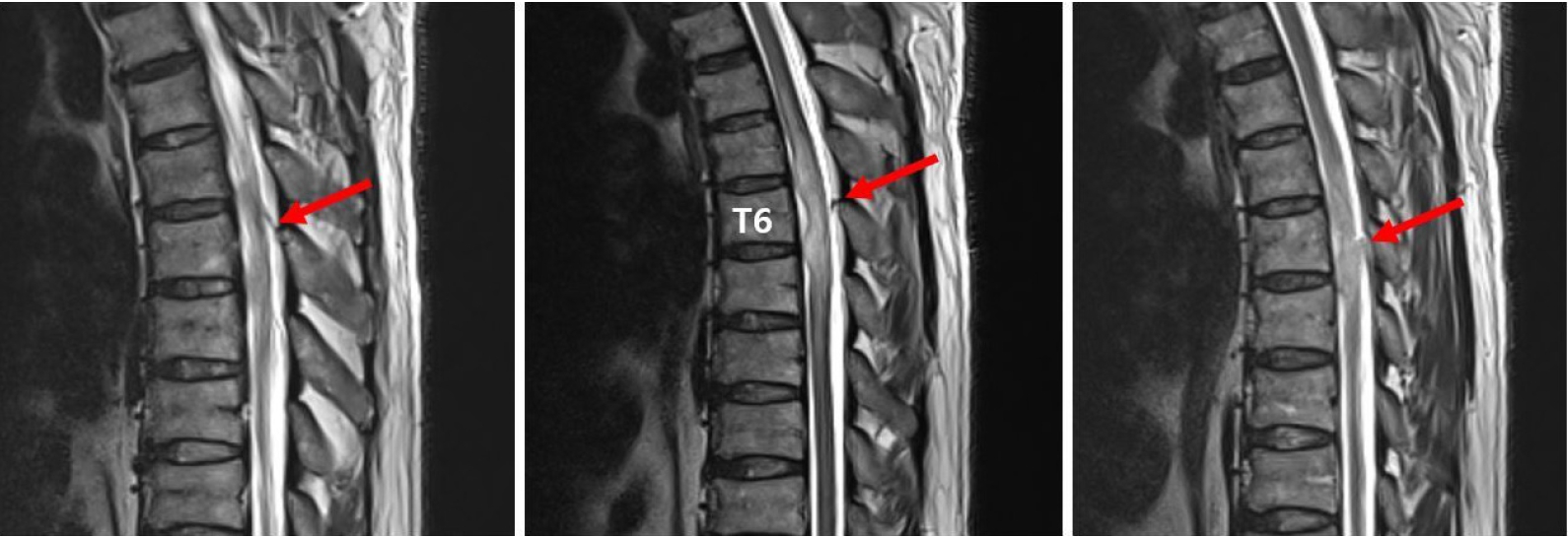
Image showing the enlargement of perimedullary vessels (arrows) at day 10 on T2 weighted magnetic resonance imaging.
Even in patients with normal immunity, ATM can occur due to VZV and result in a poor prognosis, such as complete paraplegia. Therefore, an active intervention should be considered in the presence of poor prognostic factors such as abnormal MEP and SEP. Myelitis due to SDAVF should also be considered prior to steroid pulse therapy, and any aggravation of symptoms should be noted.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.