Brachial Plexopathy with Shoulder Subluxation in a Subacute Stroke Patient
Article information
Abstract
Brachial plexus injury is a rare complication of shoulder dislocation or subluxation. A 60-year-old man was diagnosed with brachial plexopathy following a stroke. The patient reported sudden-onset left-sided weakness and was diagnosed with middle cerebral artery infarction. He was admitted to Seoul Medical Center for comprehensive rehabilitation and then was transferred to another facility after 6 weeks. Although upper limb function improved, left shoulder subluxation persisted with no meaningful symptomatic relief. Six weeks after transfer from our facility, the patient visited the outpatient department with aggravated weakness of the left upper limb. Electrodiagnostic results, along with brain and brachial plexus magnetic resonance imaging and cervical spine computed tomography, suggested a high likelihood of brachial plexopathy with evidence of a lesion affecting the whole trunk. An extended arm sling was recommended for corrective alignment, and at the 6-week follow-up visit, the patient reported moderate symptomatic relief in the upper limb. Although plexopathy is challenging to diagnose in patients with stroke, timely diagnosis and appropriate management are critical for functional recovery.
Introduction
Brachial plexus injury (BPI) represents the rarest and most severe complication of shoulder dislocation and subluxation [1]. Among patients exhibiting post-stroke symptoms, a potential mechanism for nerve injury in BPI cases is traction- or pressure-induced damage to the brachial plexus, which can occur due to extended periods of lying on the hemiplegic arm. Traction injury to the upper plexus is triggered by the subluxation of the flaccid hemiplegic shoulder, resulting in traction of the axillary, suprascapular, radial, and musculocutaneous nerves [2]. Shoulder subluxation is characterized by increased translational movement of the humeral head in relation to the glenoid fossa [3]. This leads to the caudal migration of the humeral head, compressing the axillary nerve [2,3]. Shoulder subluxation is observed in 15% to 81% of patients with post-stroke hemiplegia [3].
BPI is a potential complication in patients who experience dislocation or subluxation after stroke. Here, we present a case of brachial plexopathy that arose as a post-stroke complication.
Case Report
A 60-year-old man presented to a university hospital with sudden-onset left-sided weakness. Based on brain magnetic resonance imaging (MRI) and computed tomography (CT) angiography (Fig. 1), he was diagnosed with atrial fibrillation and right middle cerebral artery infarction. Thrombectomy was subsequently performed on the occluded artery. One month post-stroke, the patient was admitted to the Seoul Medical Center for comprehensive rehabilitation. This program included physical and occupational therapy, as well as repetitive transcranial magnetic stimulation involving 10 sessions of high-frequency (10-Hz) stimulation directed at the motor cortex on the affected side of the brain. Additionally, the patient was prescribed Lixiana (60 mg; Daiichi Sankyo, Tokyo, Japan) for secondary stroke prevention.
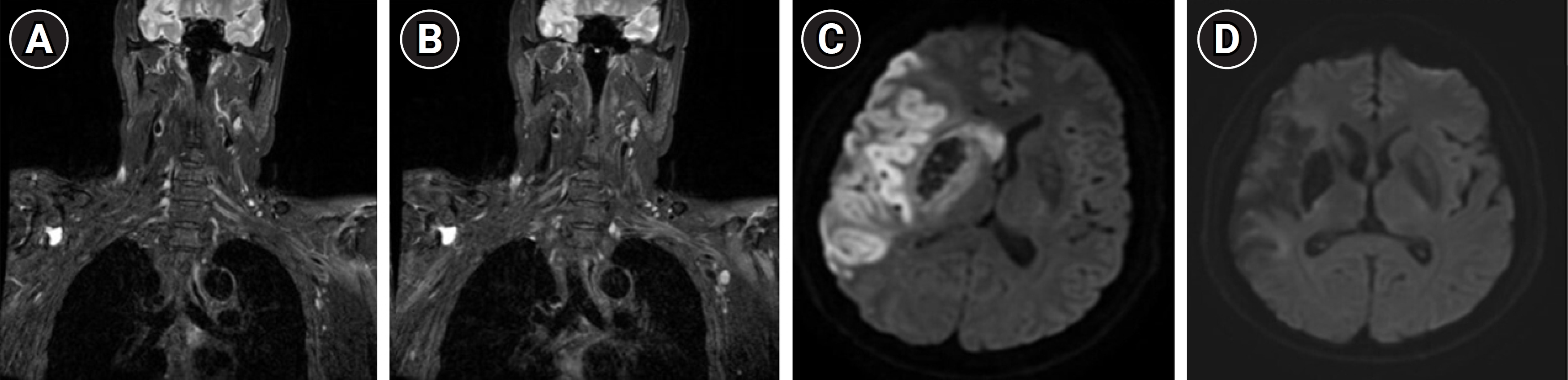
Magnetic resonance imaging of the brachial plexus and brain. (A, B) Coronal T2-weighted images of the left brachial plexus. (C, D) Brain diffusion-weighted imaging captured at the time of the infarction event (C) and at 6 weeks following the aggravation of weakness (D).
At admission, manual muscle test (MMT) results for the left shoulder, elbow, and wrist were rated as “fair,” whereas the finger flexors and extensors were assessed as “trace” (Table 1). Additionally, subluxation of the left shoulder was noted. Following rehabilitation, MMT results for the left upper extremity showed improvement (Table 1). Despite the persistence of left shoulder subluxation, the strength of the proximal muscles as measured by MMT exceeded the “fair” level; hence, an arm sling was not prescribed. The patient was transferred to another facility for further rehabilitation and was hospitalized there for 6 weeks.
Subsequently, the patient presented to our outpatient department with aggravated weakness of the left upper limb (Table 1), but no marked exacerbation in the lower limbs compared to the prior admission. He was hospitalized for comprehensive evaluation.
The patient had no history of trauma, sudden-onset pain, or infection involving the upper limb since his prior hospitalization. Brain MRI revealed no signs of acute brain infarction (Fig. 1). To differentiate between peripheral neuropathy and myopathy, electrodiagnosis (EDX), cervical spine CT, and laboratory tests were performed. Cervical spine CT revealed ossification of the posterior longitudinal ligament from C3 to C6. Laboratory tests ruled out inflammation or infection. Electromyography (EMG) and nerve conduction study (NCS) results are summarized in Tables 2, 3. Motor NCS indicated comparatively low compound muscle action potential (CMAP) amplitudes in the left median and ulnar nerves. Compared to the right side, the CMAP amplitudes on the left side were 58.1%, 36.2%, and 46.2% lower for the axillary, musculocutaneous, and radial nerves, respectively.
Sensory NCS revealed low amplitude of the bilateral median sensory nerve action potentials (SNAPs), accompanied by prolonged onset latency. Compared to the corresponding nerves on the right side, the amplitude on the left side was 49.2% lower for the lateral antebrachial cutaneous (LABC) nerve, 45.5% lower for the medial antebrachial cutaneous (MABC) nerve, and 42% lower for the radial nerve. In the lower limbs, NCS findings fell within the normal range (Table 2).
Needle EMG revealed prominent abnormal spontaneous activity in the left infraspinatus, supraspinatus, serratus anterior, biceps brachii, triceps brachii, flexor carpi radialis, extensor digitorum communis (EDC), abductor pollicis brevis, and first dorsal interosseous muscles. However, no denervation potentials were detected in the lower limbs or the cervical paraspinal muscles on either side (Table 3). Subsequently, brachial plexus MRI revealed the absence of both masses and evidence of inflammatory lesions (Fig. 1). However, plain radiography showed subluxation of the left shoulder.
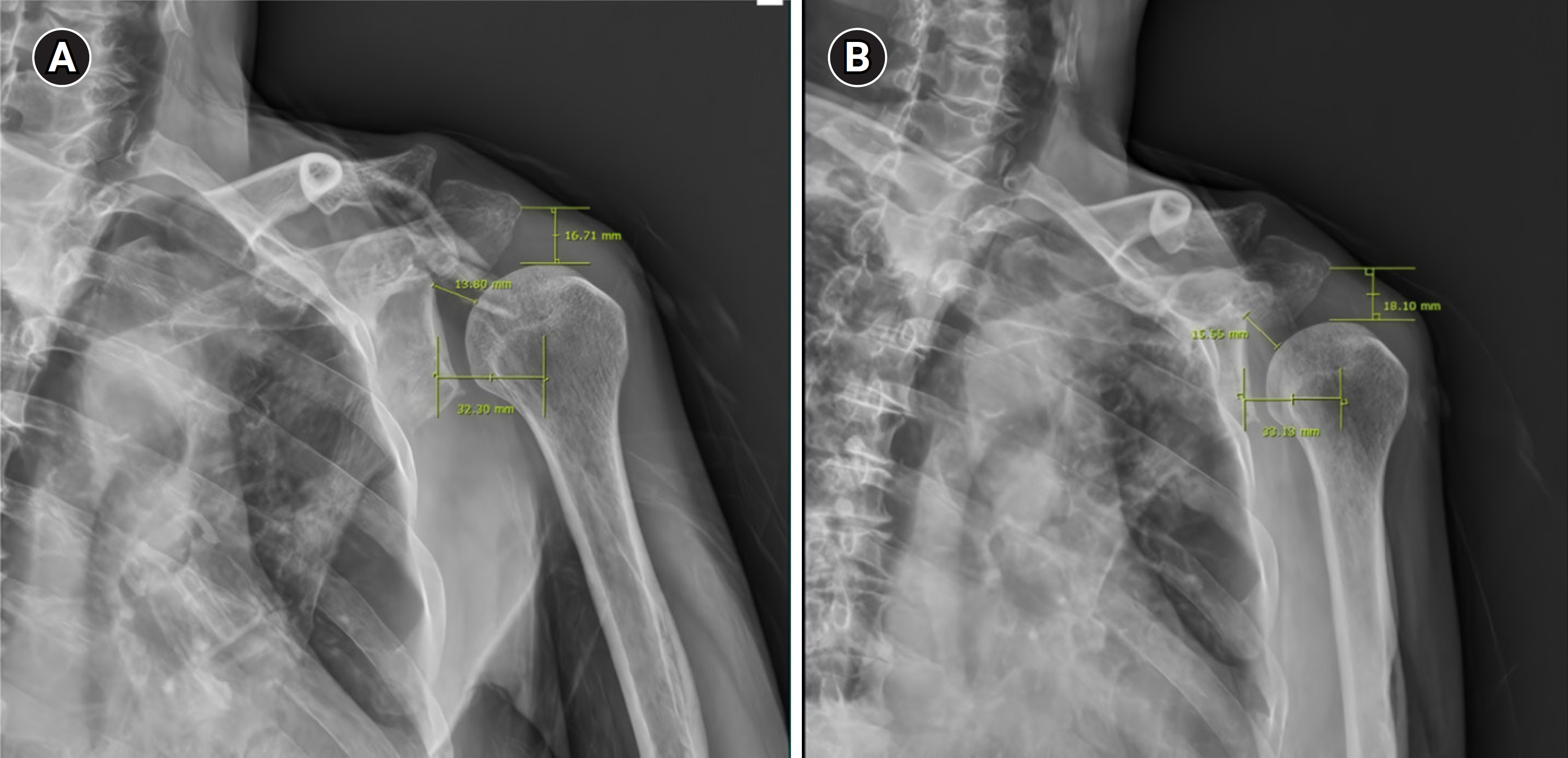
An extension-type arm sling was prescribed to correct the alignment of the shoulder joint. Five weeks after the exacerbation of weakness, we compared the shoulder joint distance before and after application of the sling (Fig. 2). The patient received conventional rehabilitation, which included physical and occupational therapy, as well as neuromuscular electrical stimulation for the shoulder girdle. Nine weeks following the aggravation of weakness, we noted an improvement in the MMT of the left shoulder, a nominal improvement in the proximal upper limb, and a slight improvement in the distal upper limb (Table 1). Sixteen weeks after the aggravation of weakness, EDX was performed. Needle EMG revealed reductions in muscle denervation and polyphasic motor unit action potentials in the deltoid, biceps, EDC, and supraspinatus muscles. NCS also indicated increases in CMAP amplitude in the left median, ulnar, axillary, and musculocutaneous nerves compared to previous measurements, along with decreases in the disparity between left and right sides for the median, LABC, MABC, and radial SNAP amplitudes (Tables 2, 3). However, despite these interventions, the left upper limb weakness persisted with minimal interval change.
Informed consent for patient information to be published in this article was not obtained because follow-up loss.
Discussion
Diagnosing BPI or other peripheral nerve issues in patients who have experienced stroke is challenging. These patients have sustained damage to the central nervous system (CNS), which complicates the task of determining whether their symptoms originate from CNS damage or peripheral nerve injury [4]. In the present case, the weakness in the patient’s left arm worsened during the recovery period. We reviewed his medical history in an effort to identify the underlying cause of this weakness. Neither this history nor the laboratory findings indicated inflammation before or after the onset of weakness. We also observed no indications of pain or trauma that would suggest a brachial plexitis lesion in the left upper limb. Brain MRI showed no evidence of new infarction or aggravated signs (Fig. 1). Similarly, brachial MRI revealed no definite plexitis or mass lesions. The combination of the patient’s history, laboratory results, and imaging findings, along with shoulder subluxation that persisted for several months post-stroke, led to the suspicion of a connection between the shoulder subluxation and BPI. The EDX findings, which included NCS results and evidence of denervation potential in muscles innervated from C5 to T1 and the serratus anterior muscle, suggested a BPI encompassing the whole trunk, with additional partial involvement at the spinal nerve root level. Follow-up EDX showed that the denervation potential in the serratus anterior muscle had resolved, suggesting that the patient was exhibiting brachial plexopathy with a whole trunk lesion, likely due to a prolonged stretching injury associated with shoulder subluxation. Even with consideration of the trans-synaptic potential, no significant differences were detected in the NCS data or denervation potentials, as measured by needle EMG, between the left and right lower limbs [5]. Consequently, the denervation potential observed in the left upper limb was attributed to a peripheral nerve lesion. To address this, a shoulder extension-type arm sling was prescribed to ensure proper positioning of the shoulder joint and prevent further aggravation of subluxation. Proper positioning or corrective alignment of the shoulder joint involves positioning the upper arm so that it can be abducted and externally rotated, with the elbow extended and the scapula protracted and elevated, all while maintaining the humeral head within the glenoid fossa [6].
Posterior subluxation and shoulder dislocation are associated with obstetric BPI [7]. Similarly, traumatic shoulder dislocation is linked to BPI [1]. However, few reports suggest a relationship between BPI and shoulder subluxation on the hemiplegic side among patients with stroke. Following stroke, the loss of the shoulder joint’s locking mechanism and upper limb weakness are attributed to the improper positioning of the humeral head, mediated by inferior displacement of the scapula [8]. Given this mechanism, it is plausible that chronic persistence of subluxation could lead to BPI, thereby impacting the hemiplegic shoulder joint.
The present case report has several limitations. Firstly, it relied solely on the patient’s recollection in determining when the initial symptom appeared. An accurate assessment of the onset of left arm weakness was hindered by the patient’s hospitalization at a different facility. Additionally, the possibility of a traction event cannot be excluded.
Second, imaging studies were not performed at the appropriate time points. Brachial MRI, brain MRI, and cervical CT were conducted approximately 6 weeks after aggravation of weakness. Furthermore, cervical MRI was not utilized to differentiate between radiculopathy and myelopathy. Despite this, neuralgic amyotrophy can generally be confirmed based on MRI findings indicating edema or focal thickening in the plexus, signal changes on T2-weighted images, and gadolinium enhancement on brachial plexus MRI [9]. These findings were not observed in the patient.
Third, the patient was experiencing hemiplegic shoulder pain, for which a bursal injection and two intra-articular injections were prescribed. These treatments could have concealed any local shoulder pain and inflammatory responses. Consequently, conditions such as brachial plexitis and neuralgic amyotrophy may have been overlooked.
Fourth, an electroencephalography could not be conducted due to the substantial time lapse between the onset of the patient’s symptoms and evaluation at the Seoul Medical Center. Furthermore, because the patient was not receiving anti-epileptic medication, it was not possible to exclude Todd paralysis resulting from partial seizure.
When a patient with no history of trauma or inflammation presents with aggravated upper limb weakness following a stroke, alternative causes, including peripheral neuropathy and BPI attributed to shoulder subluxation, must be considered. Electrodiagnostic studies serve as valuable tools for diagnosis and localization, particularly when sudden weakness takes place simultaneously with stroke recovery. Patients whose shoulder MMT scores exceed the “fair” level may benefit from the use of an arm sling to maintain proper shoulder joint alignment in chronic situations [10].
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.