Paraneoplastic Neurologic Syndrome in Small Cell Lung Carcinoma
Article information
Abstract
A 54-year-old male smoker, previously in good health, was admitted to the hospital due to a tingling sensation in his upper and lower extremities. He reported difficulty walking due to a loss of balance and numbness, leading to an initial diagnosis of chronic inflammatory demyelinating polyneuropathy. Brain and spine magnetic resonance imaging, along with needle electromyography, yielded inconclusive findings. However, a nerve conduction study indicated a length-dependent pattern of sensory-dominant polyneuropathy. A cerebrospinal fluid study did not reveal any specific findings in terms of cell numbers, proteins, or immune tests. Following hospitalization, the patient reported progressive dizziness upon standing, leading to a preliminary diagnosis of orthostatic hypotension. However, a positive anti-Hu autoantibody test, along with chest computed tomography and positron emission tomography scans, revealed a mass in the left interlobar lymph node, suggestive of lung cancer. An endoscopic biopsy confirmed the presence of small cell lung cancer (SCLC). The patient underwent chemo-radiation treatment for the SCLC and immunoglobulin therapy for sensory ganglionopathy. As a result, a definitive diagnosis of paraneoplastic neurologic syndrome was made. Although such cases are rare, our observations suggest that symptoms of dysautonomia and sensory ganglionopathy may be associated with the production of anti-Hu antibodies.
Introduction
Cancer can cause various types of lesions in both the central and peripheral nerves. These lesions may present as direct cancer invasion, immune response suppression, neuropathy, or paraneoplastic syndrome related to cancer treatment, which occurs in less than 1% of cancer patients [1]. We successfully diagnosed a male patient with small cell lung cancer (SCLC) who presented with neuropathic symptoms such as paresthesia, ataxia, and dizziness [2]. This patient was diagnosed with SCLC and paraneoplastic syndrome, specifically sensory ganglionopathy and orthostatic hypotension (OH), associated with anti-Hu antibody syndrome. This syndrome is occasionally found in patients with SCLC.
We encountered an unusual case of paraneoplastic syndrome, characterized by sensory symptoms and accompanied by dysautonomia [3,4].
Case Report
A 54-year-old man with no notable medical history was admitted to Department of Neurology, Inha University Hospital on June 15, 2021, due to a tingling sensation in his upper and lower extremities. He reported that he had been experiencing difficulty walking due to an imbalance and numbness that began a month beforehand. Furthermore, he confirmed that he had not experienced any infection-related events, such as diarrhea or fever, in the past 3 months.
The initial neurologic examination revealed normal motor power and function, cranial nerve function, and mental status. Deep tendon reflexes, including those in the knees, ankles, biceps, and triceps, were also normal. However, the patient exhibited a diminished sense of position and vibration in the limbs. Furthermore, there was a progressive decrease in sensation to pinprick and light touch moving towards the distal parts of the limbs. No pathological reflex was detected, but the Romberg test yielded a positive result. The patient's history included working as a crane operator and heavy drinking (1–2 bottles of soju/day, 3–4 times a week, for over 10 years until a decade ago). He also had a 25-year history of smoking about a pack a day.
During hospitalization, the patient consistently reported experiencing dizziness upon standing. Consequently, we conducted a tilt test to aid in the differential diagnosis of OH. The diagnosis of OH was confirmed based on his blood pressure readings, which were 129/91 mm Hg when lying down and 102/76 mm Hg when standing, accompanied by persistent dizziness. Despite implementing a treatment regimen that included compression stockings, increased salt intake, oral hydration, and an alpha-1 agonist, there was no improvement in the patient's OH symptoms. In fact, his blood pressure further declined to 80/40 mm Hg upon standing.
In his initial admission evaluation, the patient reported experiencing dizziness and a struggle to maintain balance while standing. This resulted in a score of 24 on the Berg Balance Scale, due to his inability to complete the standing balance assessment item. This suggested a potential balance impairment or an underlying neurological condition, prompting further evaluation and management.
The differential diagnosis considered cervical myelopathy, polyneuropathy, and Guillain-Barré syndrome (GBS); to distinguish between these possibilities, magnetic resonance imaging (MRI) studies for the brain and spine were performed. Brain MRI did not reveal any specific findings; however, contrast-enhanced spine MRI showed possible inflammatory lesions (Fig. 1).

Contrast-enhanced spine magnetic resonance imaging (MRI), showing thickening and prominent enhancement of the anterior/posterior nerve roots from the filum terminale. (A) An axial image at the level marked by the orange line in (B). (C) An axial image at the level marked by the orange line in (D). (B, D) Sagittal images of contrast-enhanced spine MRI.
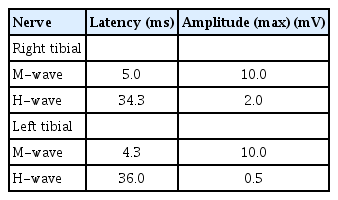
An initial nerve conduction study (NCS) revealed dominant sensory neuropathies in the upper and lower extremities (Tables 1-4), but no motor unit changes were observed on needle electromyography (Table 5). A cerebrospinal fluid (CSF) analysis revealed an albuminocytologic dissociation, characterized by an elevated protein level in the CSF without a corresponding increase in white blood cells. This phenomenon is frequently observed in certain neurological conditions, including GBS, chronic inflammatory demyelinating polyneuropathy, and some types of neuropathy. It generally indicates a chronic or subacute neuropathy rather than an acute inflammatory process. The CSF test revealed a white blood cell count of 3/mm3 (normal range, 0 to 8) and a protein level of 233.8 mg/dL (normal range, 12 to 60).

Needle Electromyographic Findings of the Right Upper Extremity and Right Lower Extremity in This Case
Based on the results of the examinations and NCS, the patient was initially treated for GBS with intravenous immunoglobulin (IVIG) and steroids during his first hospitalization in the neurology department. Subsequently, he was transferred to the rehabilitation department. An evaluation of autonomic nerve function was conducted for dizziness both before and after the treatment. The results indicated abnormal sympathetic skin responses, with no response in either the upper or lower extremities. Additionally, a heart rate response to deep breathing test suggested autonomic dysfunction related to vagal parasympathetic control.
A follow-up NCS was conducted 6 months after the initial NCS to evaluate any changes or improvements after the initial examination (Tables 6-9). The motor nerve test results showed a decrease in both latency and amplitude, indicating a deteriorating condition. The sensory nerve test revealed a more significant decline, with no response detected in any of the nerves tested.
To investigate the possibility of paraneoplastic syndrome due to a lengthy history of smoking, we examined neuronal autoantibodies. The test results did not reveal any autoantibodies, including Ri, Yo, amphiphysin, CV2/collapsing response mediator protein 5 (CRMP-5), paraneoplastic antigen Ma 2 (PNMA2), recoverin, sex-determining region Y protein-related high mobility group box 1(SOX1), and titin. However, we did identify the presence of the anti-Hu antibody. As a result, we performed a chest computed tomography (CT) and positron emission tomography (PET) scan to assess the possible presence of lung cancer associated with paraneoplastic neurologic syndrome. Chest CT revealed a mass in the left interlobar lymph node, which was further highlighted in the PET study (Fig. 2). A transbronchial needle aspiration biopsy of the mass confirmed the diagnosis of SCLC (stage T4N1M0).

(A) Computed tomography and (B) positron emission tomography images showing an enlarged left interlobar lymph node (arrow).
Based on these findings, concurrent chemo-radiation therapy was initiated for small cell carcinoma. This involved chemotherapy (etoposide/cisplatin), radiation therapy (left upper lobe, 6,000 cGy/30 sessions), and prophylactic cranial irradiation of the whole brain (2,500 cGy/10 sessions). However, despite the chemo-radiation therapy for SCLC and immunoglobulin therapy for sensory and autonomic neuropathy, there was no improvement in numbness, gait disturbance, dizziness, or syncope.
We arrived at a definitive diagnosis of paraneoplastic neurologic syndrome. Here we report a rare case in which dysautonomia (OH) manifested with sensory neuronopathy caused by anti-Hu antibody production by SCLC.
Discussion
Gait disturbance has various causes. Most commonly, it is caused by pathologies in areas of the central nervous system that control motor function, such as stroke, Parkinson’s disease, and myelopathy. Other potential causes include peripheral neuromuscular disorders like spinal stenosis, radiculopathy, entrapment neuropathies, myopathies, or foot drop resulting from peroneal nerve damage. Most peripheral nerve injuries are localized lesions that result in simple sensory changes or focal motor weakness. However, in this case, neurophysiologic studies revealed sensory-dominant, length-dependent polyneuropathy, which usually results from a metabolic or toxic cause of neuropathy, such as alcoholic, diabetic, or chemotherapy-induced neuropathy, although rarely, sensory axonal-type sensory neuropathy occurs in GBS and sensory neuronopathy is observed in paraneoplastic syndrome. Additionally, this polyneuropathy was accompanied by OH, which progressively led to autonomic dysfunction.
In the differential diagnosis of axonal-type sensory neuropathy of this patient, sensory ganglionopathy and sensory neuropathy were considered. While both conditions impact the sensory nerves, they are distinct in that each affects a specific location associated with different underlying diseases.
Sensory ganglionopathies specifically target the dorsal root ganglia or trigeminal ganglion sensory neurons. This results in a loss of balance (gait ataxia), generalized areflexia, and asymmetric sensory symptoms. When caused by cancer, as in the case of our patient, sensory ganglionopathy tends to progress more rapidly than typical sensory neuropathy. In contrast, sensory neuropathy is characterized by damage to the peripheral nerves responsible for transmitting sensory information to the brain and spinal cord. This can lead to a range of symptoms, including numbness, tingling, and pain. Diagnostic tests for sensory neuropathy may include NCS, skin biopsies, and blood tests to check for underlying conditions such as diabetes, toxic or autoimmune disorders [5]. While the clinical symptoms of these two diseases can appear similar, their underlying pathophysiology is distinct. Sensory ganglionopathy is primarily associated with autoimmune conditions like Sjögren's syndrome and cancer. Sensory neuropathy, on the other hand, can be caused by factors such as metabolic-toxic issues, compressive conditions, or ischemic lesions. As a result, the age, medical history, and other clinical characteristics of patients may also differ. In this specific case, the patient's medical history revealed slight changes in sensation, primarily in light touch and pinprick tests. These changes were thought to be due to skin damage on the legs or hands, in addition to the patient's subjective experience. The neurological examination showed a length-dependent sensory loss. Importantly, the patient exhibited new symptoms, which seemed to be a mix of tingling with pain in one area and balance and gait disturbances in another. These symptoms prevented the patient from continuing their work, suggesting the presence of two concurrent lesions.
The patient reported chronic tingling and pain in both hands and feet for several years. The loss of sensation seems to follow a symmetrical and length-dependent pattern in touch and vibration. During the initial presentation, a physical examination and NCS were conducted. The patient's history of alcohol consumption and the observed symptoms suggested a progressive form of alcohol-induced sensory neuropathy, which is characterized by a chronic progressive pattern.
However, the patient sought medical attention due to a gait disturbance, which was caused by rapidly deteriorating balance abnormalities over a 1-month period. Additionally, the patient experienced an increase in numbness in the upper extremities, particularly in the proximal region, which was more severe than previously reported. Initially, these symptoms were present in the left upper and right lower extremities. However, upon evaluation approximately 2 weeks later, numbness was also observed in the right upper and left lower extremities. These findings suggested an acute progressive pattern of sensory ganglionopathy.
The exacerbation of OH was likely also affected by sensory ganglionopathy. Moreover, a subsequent NCS carried out 6 months after the initial consultation showed an overall delay in latency and a reduction in amplitude in motor nerves. No response was detected in the left median nerve and left ulnar nerve, both of which had previously demonstrated responses in the sensory NCS. These alterations suggest a deterioration in the patient's condition. The swift decline noted in the NCS results over a few months further implies the influence of ganglionopathy.
It is important to note that our patient differed from those in the study of Lauria [6], as they did not present with areflexia. However, similarities were noted in the presence of ataxia, glove and stocking sensory changes, and autonomic impairment. Consequently, these factors posed challenges in the initial diagnosis of paraneoplastic syndrome. The pattern of elevated thresholds observed in quantitative sensory testing, as demonstrated in the study by Lauria [6], is likely mirrored in the sympathetic skin response of our patient.
Autoimmune autonomic ganglionopathy (AAG) is a rare autoimmune disorder that affects the autonomic ganglia, resulting in autonomic dysfunction. In AAG, autoantibodies specifically target the ganglionic acetylcholine receptors responsible for nerve impulse transmission. This leads to impaired autonomic function, which can manifest as symptoms such as OH, anhidrosis, gastrointestinal dysmotility, and urinary retention.
Diagnosing AAG can be challenging, since its symptoms often mimic those of other conditions. Nevertheless, the identification of autoantibodies against ganglionic acetylcholine receptors is a critical step in confirming an AAG diagnosis. The standard treatment for AAG usually includes immunomodulatory therapies such as steroids, plasma exchange, or IVIG.
Laboratory tests, including ganglionic acetylcholine receptor antibody (gAChR Ab) testing or alpha-3 ganglionic acetylcholine receptor antibody (α3gAChR Ab) testing, can help confirm the diagnosis of AAG and distinguish it from other types of autonomic neuropathy. Unfortunately, these specific tests were not conducted in this case, which is a limitation of the present case report.
We ruled out various potential causes of the subacute sensory neuronopathy, such as vitamin deficiency, toxic and metabolic disorders, chronic inflammatory demyelinating polyneuropathy, Sjögren's syndrome, infections like human immunodeficiency virus (HIV) and leprosy, and anti-myelin associated glycoprotein associated peripheral neuropathy. After excluding these possibilities, we considered the possibility of an unknown sensory neuropathy. Other tests related to rheumatism or vascular inflammation did not provide significant findings, except for the presence of anti-Hu antibodies. To further investigate, we conducted chest CT and PET scans since sensory neuropathies are commonly associated with lung cancer in patients with a long history of smoking [7].
During the patient's hospitalization, his peripheral neuropathy and OH gradually worsened. We conducted a search for similar cases using "anti-Hu syndrome" as a keyword, but only a few cases or clearly related pathological findings were found. OH is rarely found as a comorbidity associated with subacute sensory neuronopathy, and to date, only two cases of OH with albuminocytologic dissociation have been reported in patients with paraneoplastic syndrome [8].
Cancer can cause lesions in both central and peripheral nerves via direct invasion, immune responses, neuropathy, or paraneoplastic syndrome associated with cancer treatment. Paraneoplastic syndrome is observed in roughly 1% of cancer patients, and neuropathy resulting from this syndrome is diagnosed according to the criteria set forth by the PNS-Euro network consortium in 2004 and 2021 [9,10].
Paraneoplastic syndrome can involve the nervous system and can be categorized into various types depending on the areas affected. These categories include central nervous system neuropathies (such as encephalomyelitis, limbic encephalitis, subacute cerebellar degeneration, opsoclonus-myoclonus, stiff person syndrome, and motor neuron disease), peripheral nervous system syndromes (including subacute sensory neuronopathy, acute sensory motor neuropathy like GBS or brachial neuritis, paraprotein-associated neuropathy, autonomic neuropathy, and vasculitis neuropathy), and neuromuscular junction pathologies (such as myasthenic syndrome, acquired neuromyotonia, dermatomyositis, and acute necrotizing myopathy).
In this case, the presence of anti-Hu antibodies confirmed the diagnosis of SCLC through a PET study and biopsy. With the positive anti-Hu antibody and the confirmed diagnosis of SCLC, a definitive diagnosis of paraneoplastic neurologic syndrome was made according to the recognized criteria for this condition [9,10].
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.