Prolonged Limb and Respiratory Muscle Weakness Associated with Japanese Encephalitis Virus Infection: A Case Report
Article information
Abstract
Patients diagnosed with Japanese encephalitis (JE) may present with flaccid paralysis. JE has also been reported to be accompanied by anterior horn cell disease or motor axonal polyneuropathy. We report a case of a patient with JE with prolonged limb and respiratory muscle weakness who underwent electrodiagnostic studies, including a phrenic nerve conduction study, 10 months after the onset of paralysis. During that 10-month period, severe weakness of the upper and lower extremities showed no recovery, and the patient required long-term ventilator support through a tracheostomy. Nerve conduction studies and electromyography revealed chronic anterior horn cell disease with abundant denervation potentials involving the craniobulbar, cervical, thoracic, and lumbosacral segments. In addition to the nerves in the upper and lower extremities, the phrenic motor nerves showed abnormalities indicative of diaphragmatic weakness. Therefore, in patients with JE with chronic limb weakness and respiratory difficulty, thorough electrodiagnostic studies should be performed to diagnose the combination of anterior horn cell disease with encephalitis and to evaluate the condition’s severity and prognosis.
Introduction
Japanese encephalitis (JE) is caused by a virus in the family Flaviviridae [1], which can cause flaccid paralysis, even though the main pathophysiology involves the central nervous system and brain. Electrodiagnostic studies (EDXs) have demonstrated anterior horn cell disease or motor axonal polyneuropathy in such cases [2-4]. Herein, we report a case of a patient with JE who underwent EDXs 10 months after onset, revealing an anterior horn cell disease pattern involving the phrenic motor nerve, which is important for respiratory dysfunction.
Case Report
A previously healthy 59-year-old man visited our clinic 5 days after the onset of fever and headache. About 2 weeks before the onset of symptoms, the patient was bitten multiple times by mosquitoes while fishing. Before the hospital visit, limb muscle strength was normal.
Cerebrospinal fluid (CSF) showed pleocytosis (114 white blood cells/mm3) with monocyte dominance (83.3%), a high protein level (148 mg/dL), and a low glucose level (62.3 mg/dL, 42.1% of serum glucose level), suggesting viral encephalitis. Brain magnetic resonance imaging (MRI) demonstrated diffuse leptomeningeal enhancement along the cerebral sulci and cerebellar folia, consistent with viral meningoencephalitis. One day after admission, the patient’s level of consciousness started to decrease, and he was transferred to the intensive care unit (ICU) with intubation and mechanical ventilation.
During the ICU stay, antiviral agent, steroid pulse, and intravenous immunoglobulin therapies were administered, but none of those treatments were effective in preventing respiratory failure and the aggravation of motor weakness. A tracheostomy was performed, and bilevel positive airway pressure (biPAP) ventilation was applied. During this period, JE was diagnosed based on the detection of virus-specific antibodies in the CSF, and ribavirin was administered for 16 consecutive days.
Motor weakness was evaluated as grade 1 in all muscles of the bilateral upper and lower extremities based on the Medical Research Council scale when checked 40 days after the onset. When the patient was transferred to the Department of Rehabilitation Medicine, 70 days after onset, the muscle strength of the bilateral upper extremities was grade 1, and that of the bilateral lower extremities was grade 2. The serum creatine kinase (CK) level was 18 IU/L. The Montreal Cognitive Assessment (MoCA) score was 0, and the patient could obey only simple commands. In contrast to motor weakness, no abnormalities were found in sensory examinations of the extremities, body, and face. The range of motion (ROM) was limited in the bilateral ankle joints, as passive ankle dorsiflexion was limited to a maximum of 0°. A pair of ankle-foot orthoses (AFOs) was fabricated to prevent further aggravation of the bilateral ankle ROM limitation. Neither spasticity nor pathological reflexes were observed. A videofluoroscopic swallowing study showed aspiration into the airway while swallowing 2 mL of free water; moreover, the patient also tried yogurt, but failed to swallow it. Therefore, oral feeding was prohibited.
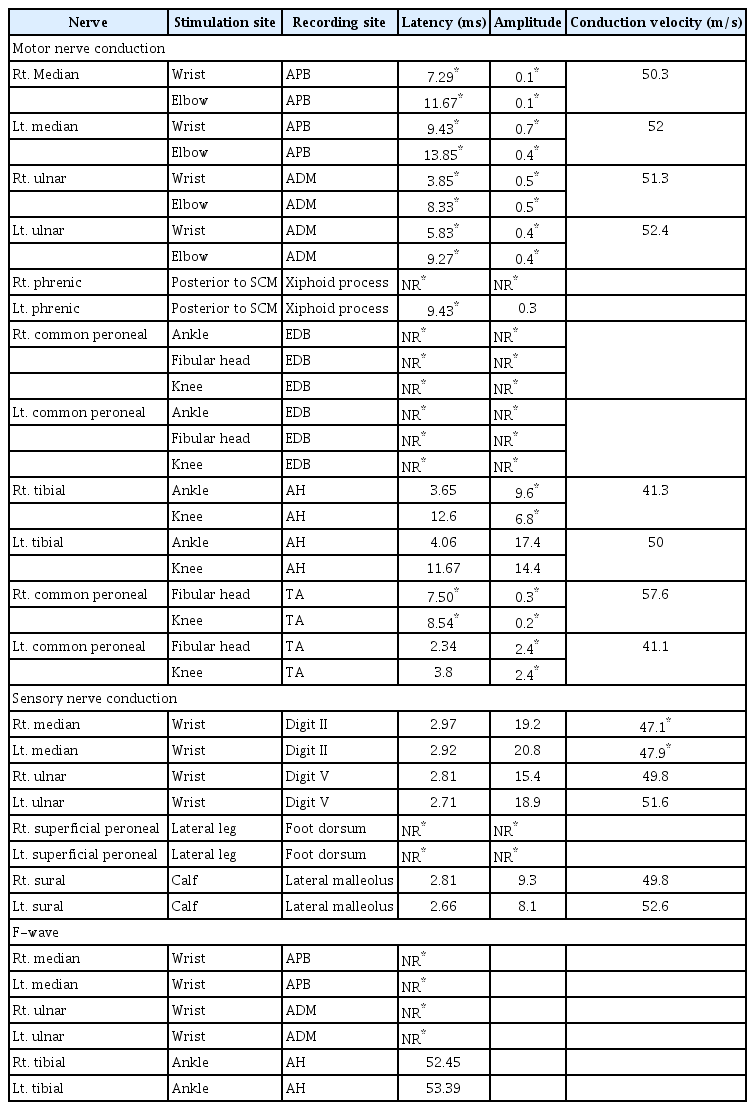
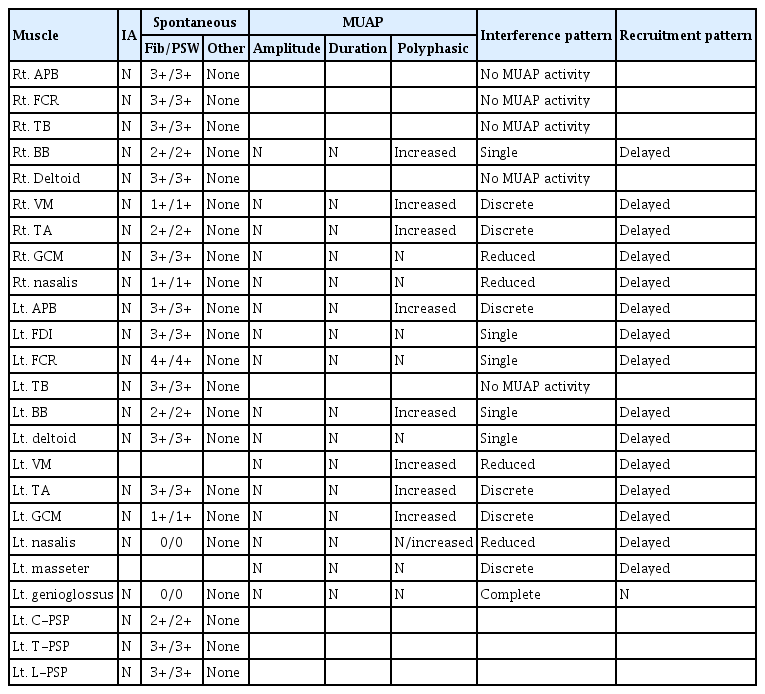
Ten months after onset, the patient’s muscle strength had not even partially recovered despite constant rehabilitation. Because the flaccid paralysis persisted for 10 months, even though the etiology was encephalitis, EDX was performed. At this time, his MoCA score was 14, and his ability to obey commands had recovered enough to perform EDX. In a nerve conduction study (NCS) (Table 1), the sensory nerve action potentials were normal, except for the bilateral superficial peroneal nerves, which showed no response, and the bilateral median nerves, which exhibited mildly decreased conduction velocities. However, in the motor NCS, both the median and ulnar nerves showed low compound muscle action potential (CMAP) amplitudes and delayed onset latencies bilaterally, but more severely on the right side than on the left. The phrenic motor NCS showed no response on the right side and delayed onset latency on the left side. The tibial nerve demonstrated a lower CMAP amplitude on the right side. The peroneal nerve studies at the tibialis anterior muscles revealed delayed onset latency on the right side, and decreased CMAP amplitudes on both sides, but with greater severity on the right side, while those conducted at the extensor digitorum brevis muscles showed no responses bilaterally. F-wave studies showed no response to bilateral median and ulnar nerve stimulation, with normal, minimal-onset latency upon tibial nerve stimulation. Electromyography (EMG) revealed abundant and diffuse denervation potentials in various muscles (Table 2). In motor unit action potential (MUAP) analyses, many muscles showed no or single MUAP interference patterns and/or chronic reinnervation potentials of neuropathic change. All muscles with at least one MUAP interference pattern showed a delayed recruitment pattern. These results suggested chronic ongoing anterior horn cell disease involving the craniobulbar, cervical, thoracic, and lumbosacral segments.
The patient underwent percutaneous gastrostomy for prolonged dysphagia and permanent weakness of pharyngeal and respiratory muscles. Although the patient could be weaned from biPAP ventilation, he experienced recurrent pneumonia and atelectasis, and oxygen was supplied when needed. Phonation was limited due to poor inspiratory strength and ventilation function, even with a fenestrated tracheostomy tube.
This case report was approved by the ethics committee at the Seoul National University Hospital Institutional Review Board (2206-205-1335) and informed consent was obtained from the patient after confirming agreement through repetitive blinking and nodding the head, and the observer who was present throughout the whole process signed the consent on behalf of the participant because of his quadriplegic state.
Discussion
In this case report, EDXs were performed in a patient diagnosed with JE with prolonged limb weakness. Prominent flaccid weakness of the limbs was found after recovery from the decreased level of consciousness, which persisted for a long time (over 10 months) after the presentation of the first symptoms. There was no improvement in motor weakness or pulmonary and swallowing function, which correlated with the EDX results of a widespread motor nerve injury, including the phrenic nerves. As permanent impairment was anticipated, he underwent tracheostomy, gastrostomy, and bilateral AFOs with portable ventilation kept ready for an emergency situation, although he did not receive ventilator support.
Previous articles have suggested JE virus infection as possibility that should be considered in the differential diagnosis of acute flaccid paralysis [2-4] because it attacks both the brain and anterior horn cells. However, there have been no reports of EDXs performed in the chronic stage, several months after onset. In our patient, an EDX, including a phrenic NCS, was performed approximately 10 months after the onset to evaluate the cause of prolonged severe limb weakness and impaired ventilation function that had hardly recovered. In the motor NCS study, low CMAP amplitudes and delayed onset latencies were observed in most of the tested motor nerves, including the phrenic motor nerves, in contrast to the sensory NCS results, which showed only mildly decreased conduction velocities in the bilateral median nerves. Furthermore, in the EMG study, abundant and diffuse denervation potentials were observed, with chronic reinnervation potentials and delayed recruitment patterns in several muscles. Therefore, chronic ongoing anterior horn cell disease with widespread distribution, including the craniobulbar, cervical, thoracic, and lumbosacral segments, was diagnosed.
Although Guillain-Barré syndrome (GBS) could be suspected when acute, flaccid limb weakness first appeared after fever, the features of prolonged severe limb weakness, CSF pleocytosis, and the EDX findings of asymmetric, non-length-dependent motor involvement patterns are not consistent with typical presentations of acute inflammatory demyelinating polyradiculoneuropathy and acute motor axonal neuropathy, which are two forms of GBS. In addition, the sensory NCS results with only mildly decreased conduction velocities of the median nerves did not correspond with acute motor-sensory axonal neuropathy, another variant of GBS, and critical-illness polyneuropathy. A severe axonal injury pattern of EMG and non-responsiveness to immune therapy also ruled out chronic inflammatory demyelinating polyneuropathy. Furthermore, the overall delayed recruitment pattern of MUAPs in the EMG study, the CK level under the lower normal limit, and the disease course with severe flaccid paralysis lasting for more than 10 months with aggravating atrophy, were not consistent with critical-illness myopathy [5].
Although superimposed bilateral carpal tunnel syndrome was suspected considering the differences in motor onset latencies between the median and ulnar nerves and mildly decreased median sensory nerve conduction velocities bilaterally, imaging studies such as ultrasonography or MRI were not performed, which is a limitation of the current study.
Diaphragmatic paresis has rarely been reported in JE [6-8]. A previous study reported a patient with JE who developed diaphragmatic paresis during the disease course, leading to delayed extubation and tracheostomy [7]. Another study reported a patient with JE who developed diaphragmatic paresis caused by longitudinally extensive transverse myelitis involving the C2–6 levels, leading to prolonged mechanical ventilation [8]. Our patient also required long-term tracheostomy and ventilator support, and diaphragmatic weakness was confirmed by abnormal phrenic NCS results and chest radiographs. Permanent phrenic nerve palsy may have resulted from an anterior horn cell disorder caused by JE. Patients who have JE with anterior horn cell involvement may require a thorough examination of pulmonary function to prevent respiratory failure and subsequent hypoxic injury.
Flaccidity has been reported to be an independent prognostic factor for poor outcomes in JE and is attributed to anterior horn cell involvement [9]. In addition, more severe anterior horn cell involvement is associated with worse recovery [10]. Therefore, EDX may have prognostic value for functional recovery in patients with JE with flaccid paralysis. In conclusion, patients with JE who present with flaccid limb weakness and respiratory difficulty should undergo thorough EDXs, including a phrenic NCS, to diagnose combined anterior horn cell involvement with a brain disorder. EDX results can provide information about the severity and prognosis of motor impairment, making it possible to plan an early intervention for compensations such as tracheostomy, ventilator support, gastrostomy, and/or AFOs.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.