 |
 |
- Search
| J Electrodiagn Neuromuscul Dis > Volume 24(2); 2022 > Article |
|
Abstract
Rectus sheath hematoma (RSH) in patients who have undergone video-assisted thoracoscopic surgery (VATS) has not been reported to date. This report describes a case of RSH in a patient with right abdominal muscle atrophy due to an intercostal nerve injury following VATS. A 61-year-old male patient complained of a bulge in the right upper abdominal quadrant after undergoing VATS. Computed tomography (CT) revealed atrophied right abdominal muscles, including the rectus abdominis, and the electrodiagnostic findings were compatible with right 8th and 9th intercostal neuropathy. The patient visited the emergency room 444 days after undergoing VATS, complaining of a left abdominal mass and pain. He had a severe cough 2 weeks prior due to underlying asthma. CT revealed an RSH in the left abdomen that shrank after 4 weeks of observation. In cases of abdominal muscle weakness due to intercostal neuropathy following VATS, the precipitating factors for RSH must be managed thoroughly.
Sensory abnormalities are generally the primary symptoms of intercostal nerve injury due to thoracic surgery and usually resolve within a few months [1]. Video-assisted thoracoscopic surgery (VATS) has a lower incidence of intercostal nerve injury than conventional thoracotomy [2]. In extremely rare cases, permanent paralysis of the rectus abdominis (RA) can occur due to intercostal neuropathy after VATS [1,3].
Rectus sheath hematoma (RSH) is an acute or chronic accumulation of blood in the sheath of the RA or within the muscle due to the rupture of an epigastric vessel or muscle. Precipitating factors for the formation of RSH include coagulation abnormalities, trauma, hypertension, pregnancy, and actions that increase intra-abdominal pressure (e.g., coughing, sneezing, vomiting, defecation, or urination) [4]. In cases of delayed RSH diagnosis, unnecessary invasive diagnostic studies or surgery may be performed, and severe bleeding may cause hypotension and even death [5].
To our knowledge, no cases of RSH after abdominal muscle atrophy due to VATS have been reported to date. Therefore, we present a case of RSH that spontaneously occurred in the contralateral abdomen of a patient who presented with symptoms of right abdominal muscle atrophy and pseudohernia due to intercostal neuropathy following VATS.
A 61-year-old male patient underwent VATS for a solitary pulmonary nodule biopsy of the right lung. VATS was performed with the three-port technique. The port insertion sites were in the right sixth intercostal space (ICS) along the posterior axillary line, the 8th ICS along the mid-axillary line, and 9th ICS along the posterior scapular line (Fig. 1A). On the first day after surgery, the patient complained of severe pain and a bulge in the right upper quadrant of the abdomen. The pain partially improved thereafter; however, the bulge did not, and the patient was discharged 1 week later. The bulge was still present at the patient’s hospital visit on the 38th day after VATS (Fig. 1A, B). It was not visible in the supine position, but protruded when the patient was sitting or standing. The patient also complained of hypoesthesia and pain in the right abdomen ranging from the xiphoid process to the umbilical level. Abdominopelvic computed tomography (CT) revealed atrophy of the right abdominal muscle, including the RA, from the level of the 9th thoracic vertebra (T9) to the first lumbar vertebra (L1), and no specific mass was observed in the muscle or subcutaneous layer (Fig. 2A).
A nerve conduction study performed 166 days after VATS revealed that compound muscle action potentials were absent in the right 8th and 9th intercostal nerves. In cases of intercostal neuropathy, there are no standardized electrodiagnostic studies other than needle electromyography; therefore, the examination was conducted using the method introduced by Pradhan and Taly [6]. Abnormal spontaneous activities of fibrillation potential and positive sharp waves were observed on needle electromyography at the supraumbilical level of the RA. The electrodiagnostic findings observed in the patient were compatible with right 8th and 9th intercostal neuropathy (Tables 1, 2).
Subsequently, electrical stimulation therapy (EST) was applied primarily to the right RA for 1 month. A study conducted by Lalingkar et al. [7] indicated that performing EST on the RA is beneficial in patients with RA muscle diastasis. EST was delivered at the following settings: frequency, 45 Hz; current, 45 mA; rise time, 1 second; stimulus time, 3 seconds; decay time, 1 second; and relaxation time, 10 seconds. The intervention was conducted for 30 minutes per day, three times a week, for 4 weeks in total. Abdominal muscle atrophy did not improve even after EST, and the patient was lost to outpatient follow-up.
On the 444th day after VATS, the patient visited the emergency room complaining of a left abdominal mass and severe abdominal pain. He had been diagnosed with asthma approximately 20 years ago and was on inhaled corticosteroids. His asthma symptoms were usually well controlled, but he had had a severe cough 2 weeks previously. At the time of the patient’s hospital visit, his vital signs were within the normal range and the cough had improved. His laboratory test results were as follows: hemoglobin, 14.6 g/dL; leukocytes, 7,470/mm3; platelets, 150,000/mm3; activated partial thromboplastin time, 26.5 seconds; prothrombin time, 10.6 seconds; and international normalized ratio, 0.92. The values were within normal ranges.
Abdominopelvic CT revealed an RSH measuring 80 × 27 × 60 mm in the left abdomen at the level of the 11th and 12th thoracic vertebrae (T11 and T12) (Fig. 2B). The medical staff recommended hospitalization; however, the patient refused and was discharged. He visited an outpatient clinic after taking tranexamic acid and acetaminophen for 3 days, and he decided to receive follow-up after 4 weeks without additional treatment after consulting with his doctor in charge. CT performed 24 days later revealed that the size of the RSH had decreased to 30 × 7 × 26 mm (Fig. 2C). However, the atrophy of the right abdominal muscles had not changed (Fig. 2B, C). Subsequently, the patient did not visit the hospital again, and long-term follow-up was not possible.
In this article, we report a case of intercostal neuropathy through an electrodiagnostic study in a patient who showed symptoms of abdominal muscle atrophy and pseudohernia after VATS; the patient later developed an RSH. The abdominal muscle atrophy did not improve, but the RSH improved after 4 weeks of follow-up with no further treatment. Cases of abdominal muscle atrophy due to intercostal neuropathy following VATS are very rare [3]. Additionally, no cases of RSH after abdominal muscle atrophy due to intercostal neuropathy have been reported to our knowledge.
Although RA rupture during severe coughing or increased abdominal pressure has frequently been reported, most of these cases have occurred in patients taking anticoagulants [8]. In the present case, no anticoagulants were administered and no obvious hemodynamic abnormalities were found in the results of the laboratory tests performed at the time of admission to the emergency room. We did not identify any other factors that could induce RSH, except for coughing caused by underlying asthma. Therefore, it is likely that the RA muscle contracted excessively due to severe coughing caused by underlying asthma, thereby damaging the RA and causing RSH [9].
According to a study by Bolser et al. [10], coughing is a defensive reflex characterized by coordinated bursts of activity in the inspiratory and expiratory muscles. Additionally, the anterolateral abdominal muscles (RA, external oblique, internal oblique, and transversus abdominis) act as a unit. However, coordination was impaired in this patient due to atrophy of the right abdominal muscle, and greater pressure was applied to the left abdominal muscle to create abdominal pressure for coughing, which is presumed to have affected RSH formation. However, as mentioned in the study by Lee et al. [5], since RSH can occur, albeit rarely, as a result of severe coughing due to underlying asthma, the possibility that RSH occurred independently without the influence of the contralateral intercostal neuropathy cannot be excluded.
In most cases, conservative treatment, such as rest, analgesic therapy, fluid resuscitation, and anticoagulation reversal, is appropriate for RSH treatment; however, transfusion, angioembolization, and surgical intervention should be considered if necessary [9]. If asthma is a precipitating factor, the general management of bronchospasm, including the appropriate use of corticosteroid medications, should be performed [5]. In the present case, the patient did not receive anticoagulants. The patient refused hospitalization, and the hematoma shrank after 24 days, even though no additional treatment was administered. Additionally, long-term follow-up was not possible. Nevertheless, this case report is meaningful in that it reports a rare clinical condition and emphasizes the need for thorough management of the precipitating factors of RSH in similar situations.
In summary, if the abdominal muscles are paralyzed because of intercostal neuropathy caused by VATS, RSH may occur due to RA damage. Therefore, in similar cases, the precipitating factors of RSH, such as respiratory diseases, must be managed thoroughly.
Fig. 1.
Photograph of the patient. The photograph shows the video-assisted thoracoscopic surgery (VATS) port insertion site (arrows) (A) and abdominal bulge (open arrows) in the right upper quadrant (A, B) on the 38th day after VATS. The port insertion sites were in the right sixth intercostal space (ICS) along the posterior axillary line, the 8th ICS along the mid-axillary line, and the 9th ICS in the posterior scapular line (A). The abdominal bulge had not changed after 1 month of electrical stimulation therapy on the 185th day after VATS (C). We received the patient’s consent form about publishing all photographic materials.
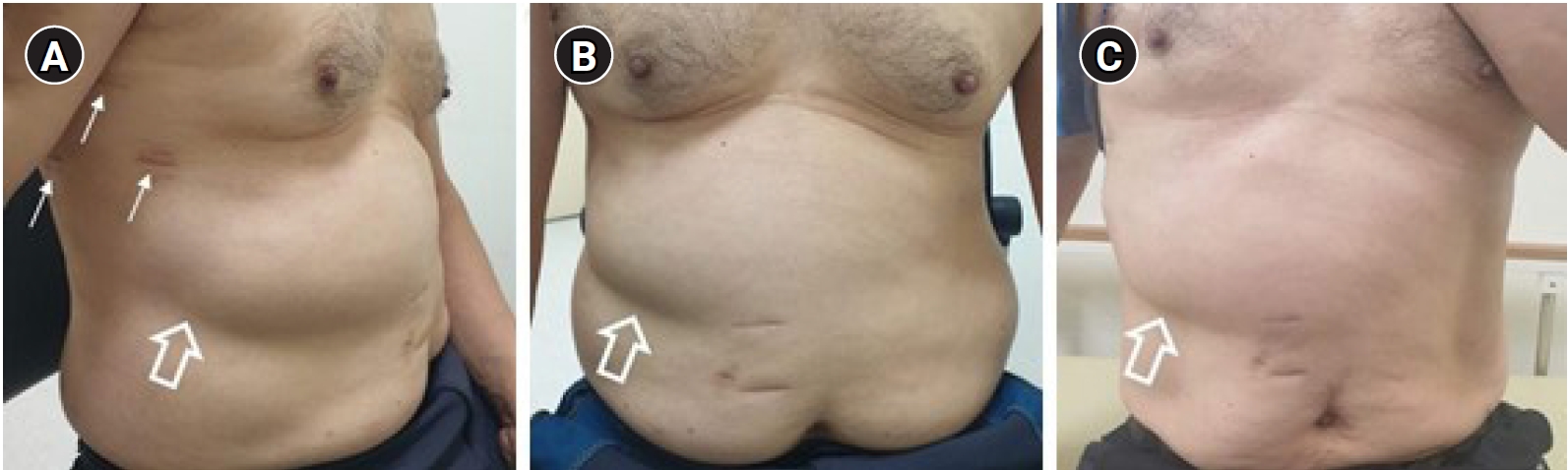
Fig. 2.
Abdominal computed tomography (CT) findings of the patient on the 38th day (A), 444th day (B), and 468th day (C) after video-assisted thoracoscopic surgery. CT revealed atrophied abdominal muscles (arrows) in the right upper quadrant (A-C) and rectus sheath hematoma (open arrows) in the left upper quadrant (B) at the level of the 11th thoracic vertebra (T11). The size of the hematoma decreased 24 days later (C).
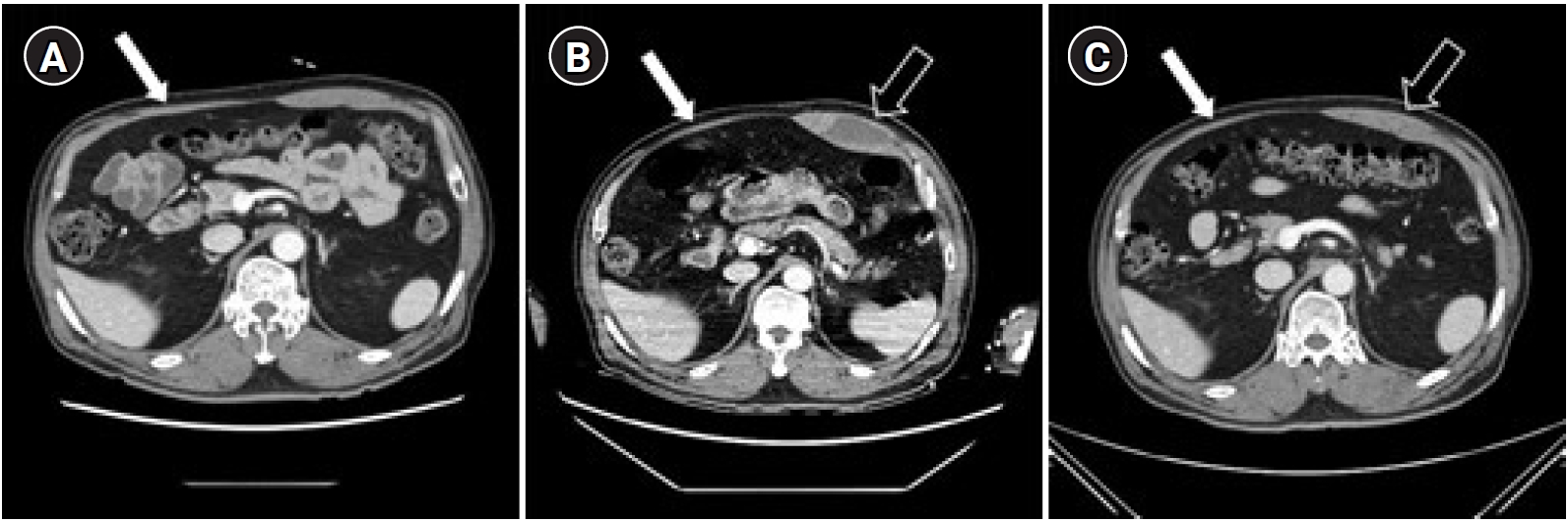
Table 1.
Results of the Intercostal Nerve Conduction Study
References
1. Cho HM, Sim HJ, Kim DH, Lim MH, Lee SK: Paralysis of the rectus abdominis muscle after a video-assisted thoracoscopic surgery. Ann Thorac Cardiovasc Surg 2018;24:40-42.



2. Miyazaki T, Sakai T, Tsuchiya T, Yamasaki N, Tagawa T, Mine M, et al: Assessment and follow-up of intercostal nerve damage after video-assisted thoracic surgery. Eur J Cardiothorac Surg 2011;39:1033-1039.


3. Wildemeersch D, Yogeswaran SK, Vyncke G, Meeus I, Wielandt T, Hans G, et al: Upper rectus abdominis paralysis after robot-assisted thoracic oncology surgery with cryoanalgesia: a rare complication. JTCVS Tech 2021;10:534-537.



4. Sogut O, Ozgonul A, Solduk L, Kose R: Rectus sheath hematoma induced by vigorous cough attacks. Am J Case Rep 2010;11:90-92.
5. Lee TM, Greenberger PA, Nahrwold DL, Patterson R: Rectus sheath hematoma complicating an exacerbation of asthma. J Allergy Clin Immunol 1986;78:290-292.

6. Pradhan S, Taly A: Intercostal nerve conduction study in man. J Neurol Neurosurg Psychiatry 1989;52:763-766.



7. Lalingkar RA, Gosavi PM, Jagtap VK, Yadav TS: Effect of electrical stimulation followed by exercises in postnatal diastasis recti abdominis. Int J Health Sci Res 2019;9:88-92.
8. DeLaurentis DA, Rosemond GP: Hematoma of the rectus abdominis muscle complicated by anticoagulation therapy. Am J Surg 1966;112:859-863.









