가슴 무딘 외상에 따른 편측 횡격막 신경 마비: 증례 보고
Unilateral Phrenic Nerve Palsy Following Blunt Chest Trauma: A Case Report
Article information
Trans Abstract
Phrenic nerve (PN) injury without a direct injury is unusual and difficult to diagnose. This case report is the first to describe the diagnosis of unilateral PN palsy following blunt chest trauma by fluoroscopic diaphragmatic movement testing (FDT) and electrodiagnostic testing. A 68-year-old man was admitted to the emergency department after a motorcycle accident. Chest radiography showed an elevated right hemidiaphragm. More than 7 months later, he experienced dyspnea on exertion and orthopnea, prompting him to visit the Department of Physical Medicine and Rehabilitation. FDT showed no movement in the right diaphragm during maximum inspiration and expiration, but the left diaphragm was intact. Electrodiagnostic testing showed absent compound motor action potential (CMAP) in the right diaphragm, but normal CMAP in the left diaphragm. We hypothesize that in patients with orthopnea symptoms after blunt chest trauma, electrodiagnostic testing paired with FDT may be useful for diagnosing diaphragm palsy.
Introduction
The phrenic nerve, the only nerve supply to the diaphragm, is both a sensory and motor nerve. Weakness in the primary respiratory muscle causes respiratory dysfunctions. The diaphragm functions as 2 separate units, a left and a right hemidiaphragm. The paresis of one hemidiaphragm can be completely asymptomatic as its counterpart and external intercostal muscles compensate for the weakened hemidiaphragm [1]. The causes of diaphragmatic palsy are divided into the following categories: traumatic, compression, neurological, infectious, and idiopathic. Cardiovascular surgery is the most common source of traumatic phrenic nerve palsy. Penetrating trauma is also another trigger of diaphragmatic muscle rupture or phrenic nerve damage [2]. As opposed to penetrating trauma, blunt trauma is caused by physical trauma of impactful force to a body part [3]. Phrenic nerve injury after penetrating trauma is identifiable, but phrenic nerve injury after chest blunt trauma is unusual, and it is often misdiagnosed as a diaphragmatic rupture.
The cause of the diaphragm elevation was difficult to identify through chest radiography and a CT scan, so we conducted an electrodiagnostic test and fluoroscopic diaphragmatic movement test (FDT). On the basis of these results, we diagnosed phrenic nerve palsy following chest blunt trauma. Many studies have shown that FDT and electrodiagnostic tests can be helpful in diagnosing phrenic nerve palsy [2]. This case report is the first to diagnose unilateral phrenic nerve palsy following chest blunt trauma by employing FDT and an electrodiagnostic test.
Case Report
A 68-year-old man was admitted to the emergency department due to a motorcycle accident. During the accident, he struck his right chest against the motorcycle’s handlebar and fell on his right side. He experienced loss of consciousness for approximately 10 minutes, but no shortness of breath or nausea. His chest radiograph showed fractures of the right 4th and 5th ribs. The trachea was deviated to the right of the midline and showed an elevated right hemidiaphragm (Fig. 1A). The patient’s chest CT scan showed multifocal subsegmental atelectasis in the left lingular segment, right middle lobe, and right lower lobe. He left emergency department under his own strength. The day after the accident, he experienced dyspnea when lifting objects, but no shortness of breath when walking or doing other daily activities. He was also unable to lay supine because of shortness of breath. To address these symptoms, 130 days after their onset, he visited the Department of Respiratory Medicine. To relieve the patient’s respiratory distress, 221 days after the onset of his symptoms, he was transferred to the Department of Physical Medicine and Rehabilitation for pulmonary rehabilitation.
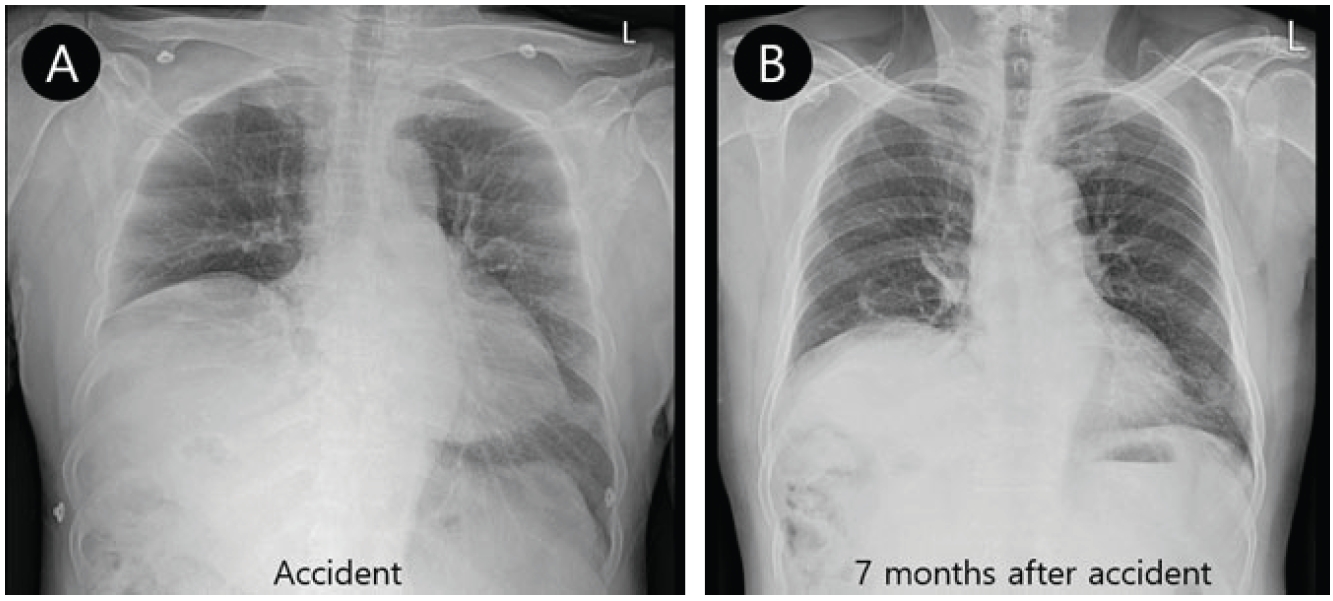
(A) Chest radiograph at admission shows that the trachea was deviated to the right of the midline and an elevated right hemidiaphragm. (B) Chest radiograph taken 228 days after onset continued to show an elevated right diaphragm, although the new radiograph reflected mild improvement since the previous chest radiograph was taken. The new radiograph also showed that the trachea was deviated to the right of the midline.
The patient was a farmer with a smoking history (30 years). His medical history was unremarkable aside from hypertension of 10 years’ duration and alcoholic liver cirrhosis of 1 year duration. The patient’s chest radiograph taken during our evaluation process prior to pulmonary rehabilitation continued to show an elevated right diaphragm and the trachea was deviated to the right of the midline (Fig. 1B). The previously observed rib fractures were no longer present. Pulmonary function tests revealed forced vital capacity (FVC) in a sitting position of 3.01 L (90% of what was predicted), and FVC in a supine position of 2.89 L (87% of what was predicted). Maximum inspiratory pressure (MIP) was 71 cmH2O (98.7% of what was predicted) and maximum expiratory pressure was 115 cmH2O (118.1% of what was predicted). These test results did not suggest diaphragm palsy, as the decrease in FVC in the supine position was not significant and MIP was almost within the normal range. Despite these results, the elevated diaphragm observed in the chest radiograph and the orthopnea symptoms prompted us to further evaluate the patient. We used fluoroscopy to observe inspiration and expiration of the diaphragm as well as an electrodiagnostic test to differentiate right hemidiaphragm palsy. Anteroposterior fluoroscopic images were acquired with a C-arm image intensifier (BV Pulsera mobile C-arm; Philips Healthcare Co., Ltd., The Netherlands) with the patient in a seated position. In the examination process, the patient was instructed to inhale deeply and exhale as much as possible. Each sequence was recorded for each hemidiaphragm. The FDT showed no movement in the right diaphragm during maximum inspiration and expiration, but normal movement in the left diaphragm (Fig. 2).
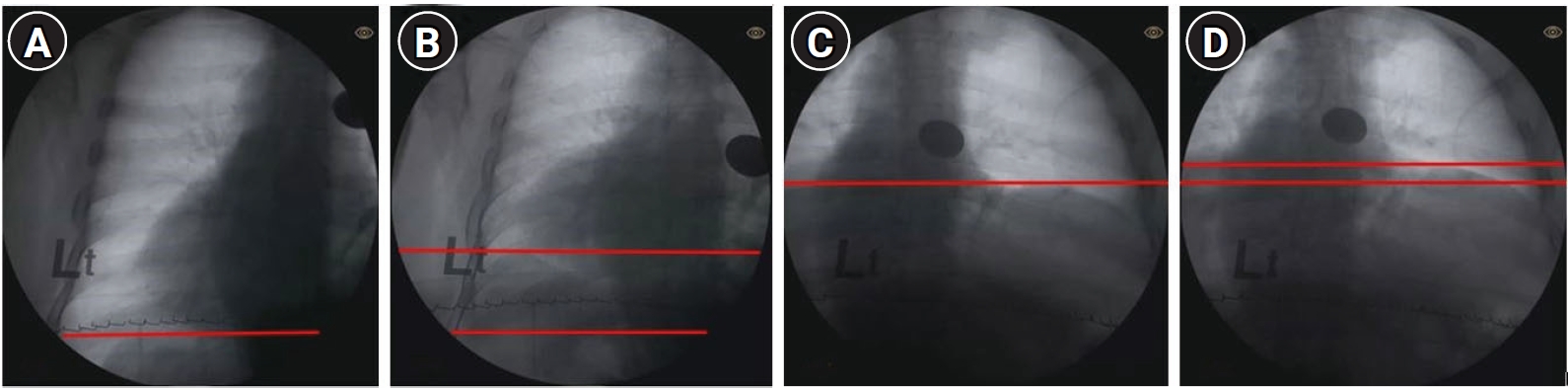
Anteroposterior fluoroscopic images with the patient in a seated position. Left diaphragm during maximum inspiration (A) and expiration (B). Right diaphragm during maximum inspiration (C) and expiration (D). The patient’s fluoroscopic sniff test showed no movement in the right diaphragm during maximum inspiration and exhalation, but normal movement in the left diaphragm.
The electrodiagnostic test for the phrenic nerve was performed on day 221 after the patient’s accident. Surface stimulation was performed above the clavicle, just lateral to the sternal portion of the sternocleidomastoid muscle. During surface stimulation, a ground electrode was placed on the sternum, an active electrode was fixed on the eighth intercostal space at the anterior axillary line, and a reference electrode was placed caudal to this on the ninth intercostal space. The results revealed that the right diaphragm had an absence of compound motor action potential (CMAP). In contrast, the left phrenic nerve’s CMAP amplitude was decreased (0.1 mV) and the CMAP latency was within a normal range (7.85 ms) (Fig. 3).
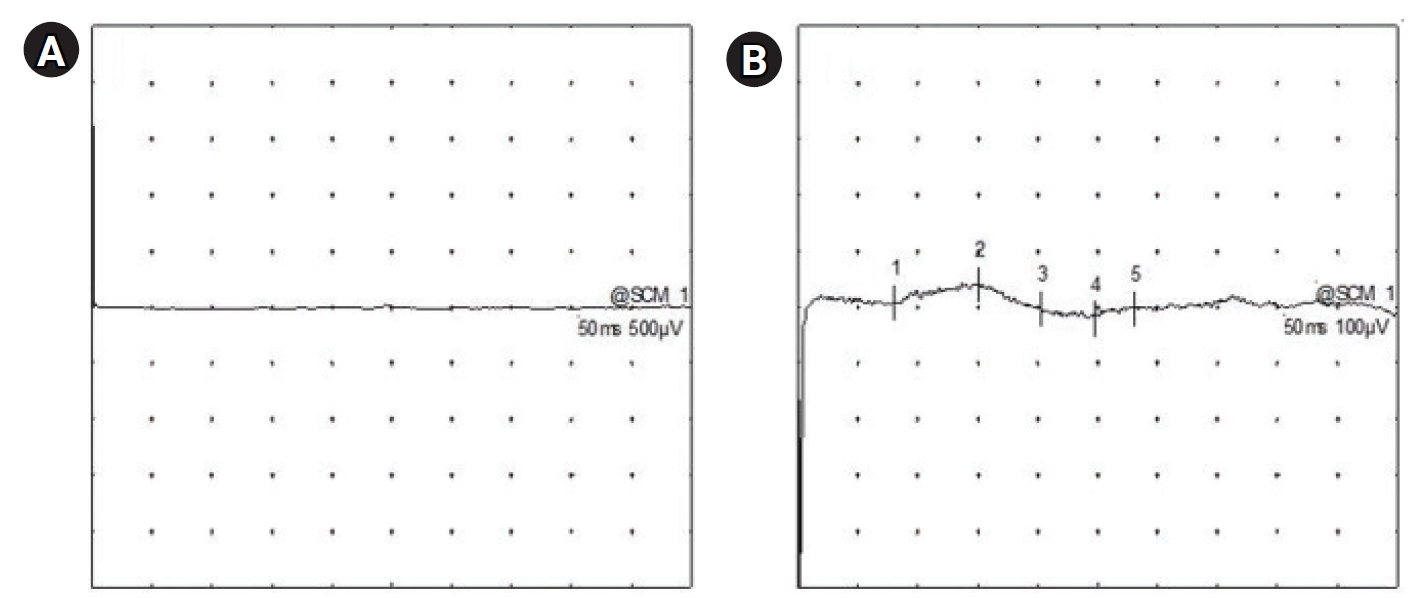
An electrodiagnostic test for the phrenic nerve was performed on day 221 after the patient’s accident. (A) The right diaphragm showed an absence of compound motor action potential (CMAP). (B) In contrast, the CMAP amplitude of the left phrenic nerve was decreased (0.1 mV) and CMAP latency of the left phrenic nerve was within the normal range (7.85 ms).
Discussion
The present case demonstrates that an electrodiagnostic test and FDT are useful in the diagnosis of phrenic nerve palsy following chest blunt trauma.
Electrodiagnostic tests of the phrenic nerve have been used to evaluate patients with respiratory failure and suspected neuromuscular disorders [4]. There was a study on the stimulation location of the phrenic nerve and this study reported that the ideal stimulation location is the supraclavicular fossa just above the clavicle [5]. Based on their study’s findings, we performed supramaximal stimulation in the supraclavicular fossa just above the clavicle. In our case, the absence of CMAPs in the right diaphragm indicated that the right phrenic nerve was severely injured.
Previous study reported that the amplitude lower limit of the left phrenic nerve was 0.25 mV and the upper limit of latency was 8.56 ms in healthy subjects [6]. CMAP onset latency is a useful parameter for detecting phrenic nerve demyelination and CMAP amplitude can be used to detect neuronal or axonal lesions [7]. The CMAP amplitude of our patient’s phrenic nerve was decreased (0.1 mV) and his CMAP latency was within a normal range (7.85 ms). This indicated that the left phrenic nerve was also damaged, but no abnormal findings were observed in the dynamic imaging test. Phrenic nerve conduction studies (NCSs), in contrast with other motor NCSs, are technically challenging as the examiner is unable to visualize the twitching of the target muscle. As a consequence, phrenic NCSs performed in isolation are associated with false negative results [8]. This aspect of the NCSs prompted us to hypothesize that dynamic imaging study might enhance diagnostic accuracy of phrenic nerve palsy.
Previous study reported that in patients with complete hemidiaphragm palsy, FVC in a supine position decreased by 15% to 20% and MIP reduced to approximately 60% [2]. These results showed that the pulmonary function test conducted in supine position was helpful in diagnosing diaphragm palsy. In our case, diaphragm palsy was difficult to detect by pulmonary function testing as the values mentioned above were near normal range. This could be due to the other respiratory muscles sufficiently compensating for the weakened diaphragm. In a previous study, although it was an animal experiment, there was a report that compensatory change in inspiratory related muscle activation occurred 14 days after onset in the presence of unilateral diaphragm paralysis [9]. In our case, we thought that the onset was long enough for compensation to occur and the symptoms in daily life were not prominent, so the decrease in pulmonary function test was not observed. It was more important to see the diaphragm’s movement through imaging tests and check the phrenic nerve’s condition.
In normal individuals, both hemidiaphragms descend caudally during tidal breathing by at least one intercostal space [2]. In our patient’s case, the left hemidiaphragm showed more than one intercostal space movement, but almost no movement in the right hemidiaphragm.
There have been rare case of a patient with phrenic nerve palsy following chest blunt trauma [10]. They observed that the patient could maintain his airway and that self-ventilation was possible; this was similar to our patient’s case. The patient mentioned in this report underwent a chest radiography and a chest CT scan. He was misdiagnosed with diaphragmatic rupture and was given surgical treatment [10]. If an electrodiagnostic test and FDT had been performed, then he would not have needed the laparotomy. Considering that phrenic nerve travels downward into the chest to pass between the heart and lungs towards the diaphragm, a nerve injury due to chest blunt trauma cannot be fully explained unless it is a direct injury. An accurate diagnosis is still important, even if chest radiographs and chest CT scans showed no evidence of a phrenic nerve injury.
To prevent early pulmonary complications, diaphragm palsy needs to be diagnosed accurately and as early as possible. Early detection of unilateral diaphragmatic paralysis also predicts a patient’s prognosis. This will help to establish proper treatment strategies.
In conclusion, finding the cause of diaphragm elevation using only chest radiographs and CT scans are insufficient. If patients complain of unexplained shortness of breath in a supine position, experience orthopnea, and show an elevated diaphragm on chest radiographs, a thorough diagnosis is needed. An electrodiagnostic test paired with FDT are useful in the diagnosis of phrenic nerve palsy following chest blunt trauma.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
