내시경적 흉부 교감신경절제술 후 발생한 외측흉근신경손상
A Rare Case of Lateral Pectoral Nerve Injury Associated with Endoscopic Thoracic Sympathectomy for Primary Hyperhidrosis
Article information
Trans Abstract
Complications may arise following an endoscopic thoracic sympathectomy; however, these are rare. Here, we report a case of isolated damage to a muscle branch of the lateral pectoral nerve in a patient who underwent endoscopic thoracic sympathectomy to treat hyperhidrosis. Eight weeks after the operation, the patient came to our clinic complaining of numbness and a tingling sensation in the left anterior chest wall, with asymmetric atrophy in the left areolar area. We performed a nerve conduction study and needle electromyography for suspected brachial plexopathy or pectoral nerve injury. These results showed an isolated left lateral pectoral nerve injury, which we attributed to the endoscopic thoracic sympathectomy. This is the first case to report an isolated lateral pectoral nerve injury after endoscopic thoracic surgery.
INTRODUCTION
The main symptom of primary hyperhidrosis is excessive sweating of the palms, axilla, and upper extremities.1 Endoscopic thoracic sympathectomy, which is considered to be the most effective treatment of primary hyperhidrosis, is known to be relatively inexpensive and uncomplicated. There have only been few reports of complications arising from surgery, such as Horner's syndrome, hemothorax, pneumothorax, Raynaud phenomenon, intercostal neuralgia, and brachial plexus injuries.2
The lateral pectoral nerve arises from the lateral cord of the brachial plexus and controls the pectoralis major muscle. Isolated injury of the lateral pectoral nerve represents a rare injury to the branch of the brachial plexus.3 According to a few reports, injury of the lateral pectoral nerve occurs due to a traction injury from trauma caused by a seat belt or sports activities.3-4 There have been no previous reports on lateral pectoral nerve injury after endoscopic surgery for hyperhidrosis.3-4 In this report, we present a case of lateral pectoral nerve injury after endoscopic thoracic sympathectomy with clinical symptoms of focal muscle atrophy, tingling sensation, and numbness.
CASE REPORT
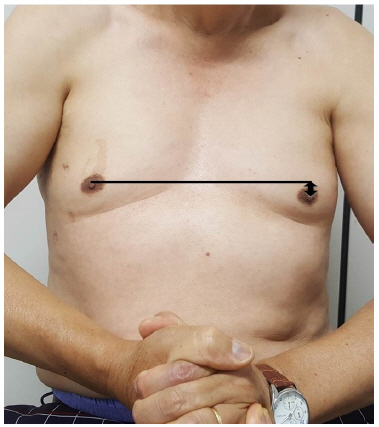
A 61-year-old male came to our outpatient clinic with chief complaints of left anterolateral chest wall tingling and numbness following a surgery 8 weeks ago. The patient had no previous issues in this region. Furthermore, he did not experience any physical activity related to heavy lifting. He previously underwent bilateral endoscopic thoracic sympathectomy due to primary hyperhidrosis. A 5mm trocar was inserted in the left 6th midaxillary line, and the left upper lobe was detached using an Endo-peanut, endograsper, and Ligarsure. An incidental laceration of left 4th intercostal artery occurred which was clipped using endoclips. On physical examination, the patient was found to have asymmetry of the areolar level due to mild to moderate atrophy of the left pectoralis major muscle (Fig. 1). The proximal portion of the manual muscle test, such as flexion, adduction, and internal rotation of left shoulder were given a grade of 3, and other motions of the left extremity were normal. Hypoesthesia around the left anterior chest wall was detected; however, sensations in other areas, including weakened shoulder, were unremarkable. No specific findings were observed on chest x-rays immediately after surgery and 4 weeks after surgery (Fig. 3.).
Chest x-rays immediately after surgery(A) and 4 weeks after surgery(B). No specific findings were observed on chest x-rays immediately after surgery and 4 weeks after surgery.
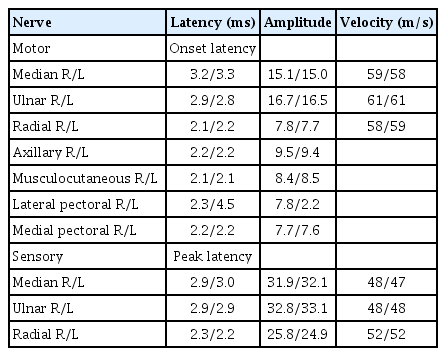
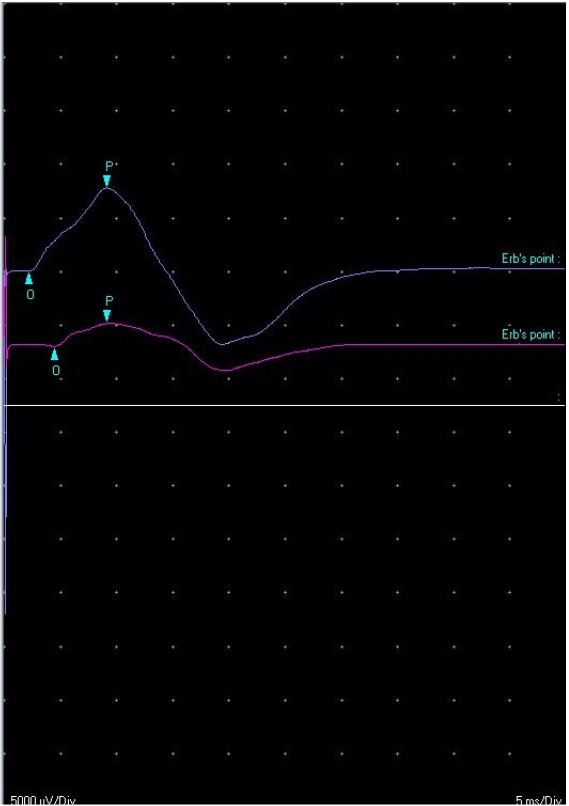
We performed an electromyography (EMG) and nerve conduction study (NCS) immediately, which suggested brachial plexopathy or pectoral nerve injury. NCS demonstrated prolonged compound muscle action potentials (CMAP), and a decreased amplitude of the left lateral pectoral nerve. CMAPs of other nerves of both upper extremities were within normal limits, including the left medial pectoral nerve. All sensory nerve action potentials studied were also within normal limits (Table 1).
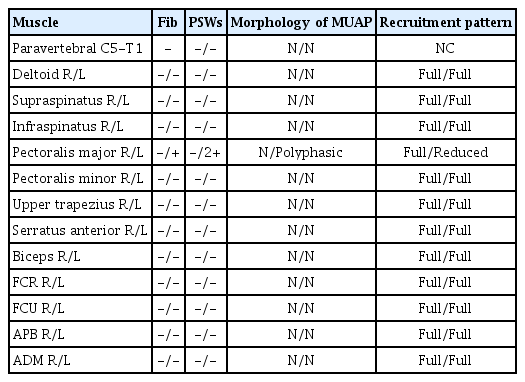
EMG of the atrophic pars clavicularis of the left major pectoral muscle revealed prominent denervation activity such as fibrillation potentials and positive sharp waves, and markedly decreased voluntary motor unit action potentials. However, EMGs of the other muscles, MUAP morphology and interference patterns were normal (Table 2). Therefore, we diagnosed the patient with an isolated injury of the left lateral pectoral nerve.
DISCUSSION
Endoscopic thoracic sympathectomy has been reported to have a 90.9% success rate for treating primary hyperhidrosis of the upper extremities since its introduction by Kotzareff in 1920.5 In addition, this minimally invasive method poses a lower risk than surgery, provides a relatively good view of the thoracic sympathetic nerve, allows operation of both sides with a single anesthesia, shortens the hospitalization period, and has an economic advantage.8
The anti-Trendelenberg posture is commonly used in patients during surgery, where the head is elevated by 20 degrees, with a 90-degree abduction of both arms, and shoulders rolled back to raise the rib cage, exposing the thoracic wall and axilla.1,9 For this posture, patients are in a supine position during abduction to postulate and elevate arms on the side to be operated. Furthermore, a raised position was taken on the left side by the patient, and the upper arm was abducted and elevated by about 150 degrees during surgery. These postures are non-physiological and can be applied to the brachial plexus for a long time. In our case, the operation lasted approximately 5 hours and 10 minutes, which is considerably longer than average surgery time.1 We inferred that a prolonged duration of operation and the position taken by the patient might have caused excessive traction injury.
We could not determine the exact cause of nerve injury since we were unable to confirm the surgical site by endoscopy. Typically, brachial plexus injuries commonly occur during surgery in the upper cervical nerve roots and the musculocutaneous nerve. The 5th and 6th cervical nerve roots mostly become damaged as the upper cervical roots are most likely pulled by abduction of the upper arm.6 However, in this case, NCS and EMG findings showed a very rare isolated lateral pectoral nerve injury. The injury of lateral pectoral nerve causes atrophy, areolar asymmetry, and weakness of the pectoralis major muscle, and anterolateral chest wall tingling and hypoesthesia. The lateral pectoral nerve arises from the lateral cord of the brachial plexus and is composed of C5, C6, and C7 branches. This runs across the lower third of the pectoralis major muscle and varies widely around the pectoralis minor muscle; therefore, pectoralis minor muscle damage can easily occur if this is not taken into account during surgery.10 Especially given that there was an incidental injury of the left 4th intercostal artery during the surgery and that the possibility of damage to the lateral pectoral nerve branch cannot be completely rule out. Considering this, the possibility of direct injury due to technical factors during surgery seems more likely than injury caused by posture traction in our case.
We suggest that the isolated injury of the lateral pectoral nerve could be caused by traction or direct injury from intraoperative procedures; therefore, this requires the attention of surgeons.7
After confirming the lateral pectoral nerve injury using the electrodiagnostic results, a follow-up was scheduled a few weeks later to investigate the changes in NCS and EMG. Unfortunately, we were unable to perform this as the patient did not return to hospital. This is a limitation of our case report.
Although endoscopic thoracic sympathectomy is a relatively safe and economical procedure, it is important to have an anatomical understanding of the surrounding nerves of the surgical area, and pay attention to the non-physiological postures, that can compress and insult these nerves. It is necessary to avoid maintaining postures which causes the nerve to be pulled for a long time, and to take precautionary measures so that treatments procedures such as drainage and hemostasis does not compress the nerve.
While there have been previous reports of various complications arising from endoscopic thoracic surgery, there have been no reports of an isolated lateral pectoral nerve injury caused by surgery. Therefore we report a rare electrodiagnostic case of isolated lateral pectoral nerve injury after surgery for treatment of hyperhidrosis. This report highlights precautionary measures that need to be considered during treatment procedures to prevent nerve injury.