Introduction
Upper-extremity deep vein thrombosis (DVT) accounts for 1% to 4% of all DVT, and the incidence is much lower than that of the lower extremities, which is reported as approximately 9.1% to 11.1% [
1]. Eighteen to sixty-seven percent of upper-extremity DVT occurs most frequently in the subclavian vein, followed by the axillary vein and the brachial vein [
2]. Risk factors for upper-extremity DVT include hypercoagulable state, intravenous catheter, trauma, malignancy, vigorous upper extremity movement, and anatomical abnormalities of the thoracic outlet structure [
3].
When DVT of upper extremities results in venous congestion, the patient complains of discomfort or changes in skin color. In severe cases, the brachial plexus is compressed and neurological symptoms such abnormal sensation and muscle weakness may occur. A case is reported that excessive flexion of the neck during cranial incision surgery causes the compression of internal jugular vein [
4], which lead to swelling of the neck and upper extremities and brachial plexus injury occurs due to venous congestion in upper extremity.
Although cases of brachial plexus injury due to venous congestion have been reported; however, a case of brachial plexus injury due to upper-extremity DVT is rare, and the extent as well as prognosis of brachial plexus injury are not well known. In this case, we report clinical manifestations and electromyographic characteristics of a patient diagnosed with brachial plexus injury due to upper-extremity DVT.
Case Report
A 66-year-old female patient with medical history of Parkinson disease and dementia presented to the emergency department with the chief complaint of deterioration of consciousness. She was admitted to nephrology department with diagnosis of acute renal failure and multiple organ failure due to bacterial sepsis, Staphylococcus capitis found on peripheral bloody culture study, and fluids and antibiotic (piperacillin/tazobactam) treatment began.
D-dimer was 5.66 mg/L fibrinogen equivalent unit (FEU) (normal range, 0-0.55 mg/L FEU) at the first laboratory result. At the 2nd hospital day, mild edema and muscle weakness were observed in right upper extremity. At the 6th hospital day, the patient showed increase in consistency with following and responding to simple verbal commands, but severe edema in whole upper extremity including shoulder girdle was noticed and muscle weakness did not show significant improvement in the right upper extremity. At the 8th hospital day, we assessed possibility of compartment syndrome, but the radial pulse was intact and pain, pallor, or abnormal sensation was not found. In manual muscle strength test, muscle strength was measured as 1 out of 5 for shoulder flexion, abduction, adduction and external rotation, elbow and wrist flexion and extension, finger flexion, extension, adduction and abduction, and skin ulceration on the lateral side of the right shoulder was observed. Imaging studies were performed to exclude central nervous system disease in brain, cervical spine, shoulder, and rib, and blood coagulation test was also performed, which all showed no abnormal findings. However, D-dimer, 9.57 mg/L FEU, was elevated in comparison to the first laboratory result. At the 14th hospital day, computed tomography (CT) venography showed multiple thrombosis in bilateral pulmonary arteries, superior vena cava, and popliteal vein. Central line was inserted, starting with anticoagulant treatment (rivaroxaban 15 mg) (
Fig. 1). The multilayer bandage treatment in right upper extremity was started at the 18th hospital day and continued before transfer to Department of Rehabilitation Medicine.
At the 30th hospital day, she was transferred to the Department of Rehabilitation Medicine for edema management. The right upper extremity edema disappeared, but muscle weakness and function in the right upper extremity did not show any significant improvement, although we could not assess sensory function in right upper extremity due to dementia. At the 47th hospital day, the electromyography was performed to assess the possibility of neuropathy in upper extremity. The sensory nerve conduction study showed that there was no sensory nerve action potential (SNAP) in right median and ulnar nerve, and the amplitude of SNAPs in right radial, lateral and medial antebrachial nerve was decreased. In motor nerve conduction study, there was no compound motor action potential (CMAP) in right median nerve, and amplitude of CMAPs in the ulnar nerve, radial nerve, axillary nerve, musculocutaneous nerve, and suprascapular nerve was decreased (
Table 1). In needle electromyography, abnormal spontaneous activity potentials were found in infraspinatus, pectoralis major, deltoid, biceps brachii, triceps, brachioradialis, flexor carpi radialis, extensor carpi radialis, first dorsal interosseous, flexor carpi ulnaris, abductor pollicis brevis and extensor indicis muscles, which led to the diagnosis of right brachial panplexopathy (
Table 2).
At 3 months after the first hospital day, the muscle strength in right upper extremity was 3 out of 5 in elbow flexion and extension. However, there was no change in shoulder flexion, abduction, adduction and external rotation, finger flexion, extension, adduction and abduction, and wrist flexion and extension, with a score of 1 out of 5, and no significant change was observed in follow-up electromyography study.
Discussion
Upper-extremity DVT is classified into primary and secondary on the basis of pathogenesis. Primary upper-extremity DVT refers either to effort thrombosis (so-called Paget-Schroetter syndrome) or idiopathic, which account for about 20% of all upper DVT. Secondary DVT results from intravenous catheter, tumors, and hypercoagulation [
5].
Although upper-extremity DVT is very rare compared to the lower extremity DVT, it can induce severe clinical symptoms that cause disability in upper extremity due to complications such as pulmonary embolism, superior vena cava syndrome, phlebitis, pain, and edema. Pulmonary artery embolism, which can lead to fatal clinical course, occurs in about one third of patients with upper-extremity DVT [
6].
Considering the clinical characteristics of DVT in upper extremity, anticoagulant administration is urgently required to relieve acute symptoms caused by venous compression and to prevent complications [
7]. The patient was diagnosed with multiple organ failure due to bacterial sepsis, which may be the potential factor to induce multiple thrombosis in bilateral pulmonary arteries, superior vena cava, and popliteal vein.
Superior vena cava occlusion secondary to thrombosis, potentially life-threatening medical emergency, may induce superior vena cava syndrome presenting with dyspnea, facial swelling, neck distension. In our case, DVT computed tomography showed multiple thrombosis in bilateral pulmonary arteries, superior vena cava, and popliteal vein, but total occlusion was not found. The multiple thrombosis may lead to venous congestion which worsened edema in right upper extremity. After multiple thrombosis were found, anticoagulant treatment and multilayer bandage were performed, which relieved edema, but the muscle weakness in right upper extremity continued after edema disappeared.
In the previous case reports about upper-extremity DVT, venous thoracic outlet syndrome occurred due to thrombus in the axillary subclavian vein [
8] or brachial plexopathy occurred secondary to endovascular stent [
9]. But specific clinical characteristics or extent of brachial plexus injury caused by venous congestion have not been reported. This case showed the brachial panplexopathy, in which all 3 branches of brachial plexus were damaged. It indicates that brachial plexus injury was more widespread, unlike thoracic outlet syndrome having injury in lower trunk of brachial plexus due to venous congestion, because it failed to detect upper-extremity DVT and initiate anticoagulation early.
Upper-extremity DVT is accompanied by pain and limitations in performing activities of daily living due to post-thrombotic syndrome, which can cause upper extremity dysfunction and significantly lower quality of life [
10]. At 3 months after the first hospital day, in this case, the upper extremity muscle strength showed no improvement except for elbow flexion and extension, and severe upper extremity dysfunction continued. Although the incidence of upper-extremity DVT is much lower than that of the lower extremities DVT, this case demonstrates that brachial plexus injury due to DVT causes more significant impairment in daily activities in lower extremity DVT. It is important that upper-extremity DVT should require early diagnosis and treatment.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Fig. 1.
Computed tomography venography showed multiple thrombi in the pulmonary arteries (arrow) and superior vena cava (arrowhead).

Table 1.
|
Nerve |
Simulation site |
Recording site |
Latency (ms) |
Amplitude |
Conduction velocity (m/s) |
|
Motor nerve conduction |
|
Rt. median |
Wrist |
APB |
NR |
NR*
|
NA |
|
Elbow |
APB |
NR |
NR*
|
NA |
|
Rt. ulnar |
Wrist |
ADM |
1.85 |
0.3 |
NA |
|
Elbow |
ADM |
NR |
NR*
|
NA |
|
Rt. MSC |
EP |
Biceps |
5.45 |
0.2*
|
NA |
|
Lt. MSC |
EP |
Biceps |
4.55 |
6.7 |
NA |
|
Rt. axillary |
EP |
Deltoid |
3.2 |
0.4*
|
NA |
|
Lt. axillary |
EP |
Deltoid |
4.2 |
3.3 |
NA |
|
Rt. radial |
Upper arm |
EIP |
5.55 |
2.7*
|
NA |
|
Lt. radial |
Upper arm |
EIP |
4.95 |
7.4 |
NA |
|
Rt. suprascapular |
EP |
IF |
5.45 |
0.3*
|
NA |
|
Lt. suprascapular |
EP |
IF |
4.35 |
2.4 |
NA |
|
Sensory nerve conduction |
|
Rt. median |
Wrist |
Hand |
NE |
NE*
|
NA |
|
Rt. ulnar |
Wrist |
Hand |
NE |
NE*
|
NA |
|
Rt. radial |
Wrist |
Hand |
2.4 |
16.5*
|
NA |
|
Lt. radial |
Wrist |
Hand |
2.5 |
43.5 |
NA |
|
Rt. LABC |
Elbow |
Elbow |
1.7 |
4.4*
|
NA |
|
Lt. LABC |
Elbow |
Elbow |
1.95 |
23.4 |
NA |
|
Rt. MABC |
Elbow |
Elbow |
2.1 |
7.3*
|
NA |
|
Lt. MABC |
Elbow |
Elbow |
2.1 |
12.3 |
NA |
Table 2.
|
Muscle |
IA |
Spontaneous |
MUAP |
Recruitment pattern/IP |
|
Fib/PSW |
Other |
Amplitude |
Duration |
Polyphasic |
|
B. C5-T1 PSP |
Poor resting |
|
|
Rt. SERR |
N |
None |
None |
N |
N |
N |
Disc*
|
|
Rt. RHOMB |
N |
None |
None |
N |
N |
N |
Single*
|
|
Rt. INFR |
N |
1+/2+ |
None |
N |
N |
N |
Single*
|
|
Rt. PEC |
N |
2+/0*
|
None |
N |
N |
N |
Single*
|
|
Rt. DELTOID |
N |
2+/2+*
|
CRD1+*
|
N |
N |
N |
Single*
|
|
Rt. BB |
N |
0/1+*
|
None |
N |
N |
INC*
|
Disc*
|
|
Rt. TRICEPS |
N |
0/1+*
|
None |
N |
N |
N |
Disc*
|
|
Rt. BR |
N |
0/1+*
|
None |
N |
N |
N |
Disc*
|
|
Rt. FCR |
N |
0/4+*
|
None |
N |
N |
N |
Disc*
|
|
Rt. ECRB |
N |
0/2+*
|
None |
N |
N |
N |
Single*
|
|
Rt. FDI |
N |
0/3+*
|
None |
N |
N |
N |
Single*
|
|
Rt. FCU |
N |
0/3+*
|
None |
N |
N |
N |
Single*
|
|
Rt. APB |
N |
0/3+*
|
None |
N |
N |
N |
Single*
|
|
Rt. EIP |
N |
0/3+*
|
None |
N |
N |
N |
Single*
|
References
1. Sajid MS, Ahmed N, Desai M, Baker D, Hamilton G: Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol 2007;118:10-18.


2. Bernardi E, Pesavento R, Prandoni P: Upper extremity deep venous thrombosis. Semin Thromb Hemost 2006;32:729-736.

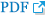
4. Shimizu S, Sato K, Mabuchi I, Utsuki S, Oka H, Kan S, et al: Brachial plexopathy due to massive swelling of the neck associated with craniotomy in the park bench position. Surg Neurol 2009;71:504-508.

5. Mustafa J, Asher I, Sthoeger Z: Upper extremity deep vein thrombosis: symptoms, diagnosis, and treatment. Isr Med Assoc J 2018;20:53-57.

6. Joffe HV, Goldhaber SZ: Upper-extremity deep vein thrombosis. Circulation 2002;106:1874-1880.

9. Liu Y, Wu Z, Huang B, Yang Y, Zhao J, Ma Y: Venous thoracic outlet syndrome secondary to arterial stent implantation: a case report. Medicine (Baltimore) 2019;98:e17829.

10. Kahn SR, Elman EA, Bornais C, Blostein M, Wells PS: Post-thrombotic syndrome, functional disability and quality of life after upper extremity deep venous thrombosis in adults. Thromb Haemost 2005;93:499-502.











