 |
 |
- Search
| J Electrodiagn Neuromuscul Dis > Volume 22(2); 2020 > Article |
|
Abstract
We report a patient with clinical presentation of Lambert-Eaton myasthenic syndrome (LEMS) and paraneoplastic cerebellar degeneration (PCD) prior to the diagnosis of small cell lung cancer (SCLC). A sixty-year-old man suffered from subacute dizziness, ataxia, dysarthria, ptosis, and proximal weakness of limbs. A repetitive nerve stimulation (RNS) test showed a decremental responses at low-frequency (LF) stimulation and an incremental responses at high-frequency (HF) stimulation, suggesting the pattern of LEMS. There was no evidence of a specific lesion on brain magnetic resonance imaging (MRI), so we concluded his cerebellar ataxia, nystagmus and dysarthria were caused by PCD. We suspected that the PCD was coexistent with LEMS as a paraneoplastic neurological syndrome (PNS) associated with SCLC. Initial screening for cancer was negative; however, SCLC was detected through computed tomography of the thorax at four-months follow-up. Early recognition of PNS and repeated screening tests can lead to a timely diagnosis and earlier initiation of treatment of SCLC.
Paraneoplastic neurological syndrome (PNS) can affect the central nervous system (e.g., paraneoplastic cerebellar degeneration [PCD]), and the neuromuscular junction (e.g., Lambert-Eaton myasthenia syndrome [LEMS] and myasthenia gravis [MG]) or the peripheral nervous system (e.g., autoimmune neuropathy) [1]. A small percentage of patients with small cell lung cancer (SCLC) have PNS, of which the most frequent type is LEMS [2]. Here, we report a case where the symptoms of PNS arose before the diagnosis of SCLC, and we emphasize that, there is a need to pay attention to these patients with PNS. Repeated cancer screening tests, such as computed tomography (CT) or F-fluorodeoxyglucose- positron emission tomography (FDG-PET) scans, are recommended for a patient with limb weakness due to a neuromuscular junction disorder (especially the LEMS type) [2]. Early diagnosis of cancer allows for earlier initiation of anti-cancer therapy, and thereby improves survival. We experienced a patient who presented initially with LEMS, and cerebellar symptoms and was finally diagnosed with PNS associated with SCLC.
A sixty-year-old male patient, previously a civil engineer, was admitted to the neurology department because of ataxia, dysarthria, ptosis, dizziness progressive weakness in the proximal limbs, and difficulty walking for two-weeks. On physical examination, he exhibited spontaneous nystagmus, hypo-reflexia, and disturbances in standing and tandem walk test. Orthostatic hypotension was diagnosed by head-up tilt test. Brain magnetic resonance imaging (MRI) revealed no specific abnormal findings. Serology results for antibodies related to non-cancer-associated syndrome (AChR-Ab, anti-GQ1b IgG and IgM, and anti-MuSK Ab) and paraneoplastic antibodies (anti- Hu, anti-Ri, anti-Yo, anti-amphiphysin, anti-CV2, anti-recoverin, anti-SOX1, and anti- PNMA2) were negative. The thyroid function test results were normal. Initial repetitive nerve stimulation (RNS) testing at the neurology department revealed that a compound muscle action potential (CMAP) amplitude in the abductor digiti minimi (ADM) was within the normal reading, and there was a 24% decrement in the response of the ADM on low-frequency (LF) (3Hz) RNS. The CMAP amplitude of the ADM did not increase after brief exercise. These results suggested the pattern of MG (Table 1). A chest CT was performed to look for signs of thymoma, and showed a mass in the anterior mediastinum, suggesting thymoma without evidence of cancer. The patient underwent thymectomy, but a subsequent pathological examination revealed only a thymic cyst. He was treated with a combination of oral prednisolone (60 mg/day for 7days, tapering by 10 mg/day to achieve a 10 mg/day maintenance dose over a total 15-week course) and pyridostigmine (180mg/day maintenance dose) under the impression he had MG.
However, despite the addition of intravenous immunoglobulin therapy for 7 days, the effect of the medical treatment was unclear. His bilateral proximal weakness, truncal ataxia, nystagmus, and gait disturbances continued for several months. The patient was unable to ambulate even with a walker. Therefore, we re-evaluated his neurophysiology study at four months after the initial RNS test. A motor nerve conduction study (NCS) indicated slightly delayed latencies and significant low amplitudes, but his conduction velocities were preserved. His sensory NCS results were normal. On electromyography, proximal and distal limb muscles showed motor units changes with short durations and variable amplitudes. The second RNS test showed a 24% decremental response in the ADM on LF (3Hz) stimulation at rest and the CMAP amplitude of left ADM in post-exercise (immediately) was increased by 260% compared to the baseline. The CMAP of left ADM exhibited an 80.2 % incremental response under high-frequency (HF) stimulation (20 Hz). The 20 Hz HF-RNS test could not be sufficiently performed, but the first 10 potentials demonstrated a tendency to facilitation. These findings were consistent with the pattern of LEMS (Table 1, Fig. 1). Although the patient refused a test for Voltage-gated calcium channels (VGCC) antibody, we considered that he had LEMS based on his clinical symptoms and electrophysiologic test findings.
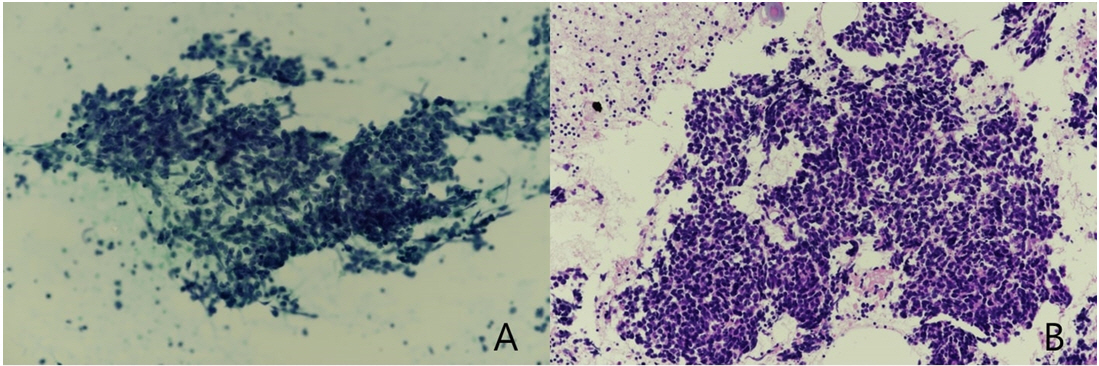
We conducted chest CT and FDG-PET scans to screen for lung cancer, but no significant lesions were detected (Fig. 2A). Considering the cerebellar signs and ptosis, and the proximal motor weakness with fatigue, we strongly suspected PNS manifesting with PCD, coexisting with LEMS associated with lung cancer. At a four-month follow-up after the initial screening, we performed chest CT and FDG-PET scans again, which showed significant lymphadenopathy in the right paratracheal area (Fig. 2B). The pathology of endobronchial ultrasound bronchoscopy fine needle aspiration finally confirmed a mediastinal lymph node metastasis of SCLC (Fig. 3A, B). Bone scanning showed no evidence of distant metastasis. The patient has since received chemotherapy and radiation therapy. At one year follow-up, abdomen CT, brain MRI, and bone scan results showed no distant metastasis. Presently, he can ambulate with a cane after gait and balance training for several months.
PNS can manifest in various forms including PCD and as neuromuscular junction disorders (e.g., LEMS and MG) [1]. LEMS and PCD can be present together as PNS in rare cases associated with SCLC.3 In one study, at least 16% of patients with SCLC and PCD had LEMS [4]. Some reports indicated that 41% of patients with PCD and SCLC had P/Q type VGCC antibodies, which are presumed to have a role in the pathogenesis of the cerebellar dysfunction, and 43% of these patients had LEMS [5]. In patients with SCLC, PCD may occur with or without Hu anti-neuronal antibodies (HuAb) [4].
The results of RNS tests are important in diagnosing LEMS. The classical pattern of LEMS shows a low compound muscle action potential (CMAP) amplitude at rest, a 10-15% decremental response in LF-RNS, and a >100% incremental response in HF(10-30Hz)-RNS [5]. In the initial RNS test of our patient, the CMAP amplitude of the left ADM at rest was within normal and did not increase at post-exercise. This pattern of RNS suggested MG, so HF-RNS was not performed at that time. Although the patient was treated under the impression of MG, the possibility of other diseases was suspected, because he was negative for AChR-Ab and anti-MuSK Ab and he showed little response to pyridostigmine. On the second RNS test at 4 months follow-up after the initial RNS, this case showed a low CMAP amplitude (3.1 mV) which increased by 260 % (11.2 mV) after brief exercise. We considered that the CMAP amplitude of ADM at rest gradually decreased as LEMS progressed. If symptoms of LEMS are mild or in an early stage, it is not easy to differentiate it from MG because the CMAP amplitude can be normal, and it does not exhibit the characteristic post-exercise facilitation [6]. Therefore, serial follow-up studies with high rate RNS are important when treating patients with LEMS.
The clinical symptoms of PCD can manifest with ataxia, dizziness, nystagmus, dysarthria, and dysphagia [7]. The criteria of PCD are: no evidence of significant cerebellar atrophy more than the expected on MRI, subacute neurologic symptoms within three months, and a Rankin score of at least 3 (moderate disability; requiring some help) caused by the cerebellar syndrome. Our patient was unable to walk independently due to ataxia, his cerebellar symptoms started within two weeks, and his brain MRI was negative for other causes. Therefore, the cerebellar symptoms in our case were considered to be caused by the PCD.
A repeated screening of radiologic imaging such as chest CT and FDG-PET is important for early recognition of SCLC in LEMS and PCD. The CT scanning of the thorax has revealed a sensitivity of 83% at primary screening and 92% overall in patients with LEMS [8]. FDG-PET can help in the diagnosis of lung cancer in patients with a negative CT thorax result at primary screening [2]. If suspicion of a malignancy remains high, the first repetition of screening should be performed after 3 or 4 months [2]. According to a large cohort study, two years of screening is sufficient for patients with LEMS [8].
A limitation of this report was that we did not perform a VGCC antibody test due to the patientŌĆÖs refusal. Patients with LEMS who are positive for the P/Q-type VGCC antibody showed a prolonged survival [5]. The results of the VGCC antibody test would have been helpful for diagnosis and prognostication in this case.
In conclusion, we experienced a case with progressive weakness of the limbs, ataxia, ptosis, and dysarthria as the initial symptoms of lung cancer without respiratory symptoms. These symptoms were found to be diagnosed with LEMS and PCD. Although initial screening for lung cancer was negative, small cell lung cancer was finally diagnosed through repeated screening. Therefore, early recognition of PNS and repeated screenings would improve the speed of diagnosis and allow for earlier initiation of treatment of the SCLC.
Fig.┬Ā1.
The second follow up repetitive nerve stimulation (RNS) test in the patient with Lambert-Eaton myasthenic syndrome (LEMS) (A) Baseline: the decreased initial compound muscle amplitude potential (CMAP) amplitude of the left abductor digiti minimi (ADM) and the decremental responses by 24% during 3Hz low-frequency (LF) stimulation (B) immediately post-exercise: Increased the CMAP amplitude of left ADM by 360 % compared to the baseline and the decremental responses by 17.5% during 3Hz LF stimulation C) 20hz high-frequency (HF) RNS test : incremental responses by more than 80% between 1st and 10th responses.
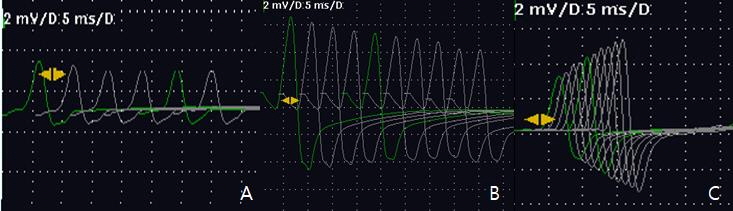
Fig.┬Ā2.
(A) Initial FDG-PET showed no abnormal lesion. (B) FDG-PET at four-month follow-up evaluation showed newly appeared lymphadenopathy (white arrow) in the right upper paratracheal area.

Fig.┬Ā3.
Cytologic findings of lymph node by endobronchial ultrasound bronchoscopy-fine needle aspiration. (A) Smear (PAP Stain) (B) Cell block (H&E stain). Microscopic findings showed the clusters of round to oval cells with finely dispersed nuclear chromatin, nuclear molding with scanty cytoplasm and apoptotic bodies.
Table┬Ā1.
Initial RNS Test and Follow-up Test after 4 Months
References
1. Pelosof LC, Gerber DE: Paraneoplastic syndromes: An approach to diagnosis and treatment. Mayo Clin Proc 2010;85:838-854.



2. Verschuuren JJGM, Titulaer MJ, Wirtz PW, Willems LNA, van Kralingen KW, Smitt PAES: Screening for Small-Cell Lung Cancer: A follow-up study of patients with lambert-eaton myasthenic syndrome. J Clin Oncol 2008;26:4276-4281.


3. Clouston PD, Saper CB, Arbizu T, Johnston I, Lang B, Newsom-Davis F, et al: Paraneoplastic cerebellar degeneration. III. Cerebellar degeneration, cancer, and the Lambert-Eaton myasthenic syndrome. Neurology 1992;42:1944-1950.


4. Clouston PD, Saper CB, Arbizu T, Johnston I, Lang B, Newsom-Davis F, et al: Small-cell lung cancer, paraneoplastic cerebellar degeneration and the lambert-eaton myasthenic syndrome. Brain 1997;120:1279-1300.


5. Wirtz PW, Lang B, Graus F, van den Maagdenberg AM, Saiz A, de Koning Gans PA, et al: P/Q-type calcium channel antibodies, Lambert-Eaton myasthenic syndrome and survival in small cell lung cancer. J Neuroimmunol 2005;164:161-165.


6. Joo IS: Diagnosis of myasthenia gravis and other neuromuscular junction disorders. Korean J Neuromuscul Disord 2014;6:7-11.
-
METRICS

-
- 0 Crossref
- Scopus
- 2,646 View
- 33 Download
- Related articles in J Korean Assoc EMG Electrodiagn Med
-
Paraneoplastic Neurologic Syndrome in Small Cell Lung Carcinoma2023 August;25(2)





